How to dectet Mitochondrial Permeability Transition Pore ?
Background
Mitochondrial membrane permeability transition pores (MPTP) are nonspecific channels located in the inner and outer membranes of mitochondria that appear to be involved in the release of mitochondrial components during cell death. MPTP switching significantly changes mitochondrial permeability and mitochondrial membrane potential. This sustained pore activation is caused by mitochondrial Ca2+ overload, mitochondrial glutathione oxidation, elevated mitochondrial reactive oxygen species levels, and other pro-apoptotic conditions. MPTP is closely related to cell survival and apoptosis, and the detection of MPTP is also permeated in many fields, such as ischemia/reperfusion, tumor, aging, neurodegeneration, etc. We developed an imaging microanalysis and flow cytometry kit for detecting mitochondrial transition pore opening. We developed an both can conduct imaging microanalysis and flow cytometry kit for detecting mitochondrial transition pore: Mitochondrial Permeability Transition Pore Assay
Principle
Under normal conditions, a high concentration of Ca2+ is maintained in mitochondria to facilitate the normal excitation, contraction and relaxation of muscles, and reduce the damage of the ultrastructure of skeletal muscle caused by the increase of Ca2+ concentration in cytoplasma. At the same time, it is necessary for the function of sensitive mitochondrial enzymes (C3H6O acid dehydrogenase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase).
Most mitochondria have a point of contact with the endoplasmic reticulum and establish a solid connection. Studies have shown that after the release of Ca2+ in the endoplasmic reticulum, part of it enters the cytoplasm, causing the increase of intracytoplasmic Ca2+ concentration, and part of it is recruited and absorbed by neighboring mitochondria. At this time, the low-conductivity permeability conversion pore switches back and forth between opening and closing. When the cell is damaged, the permeability of the low-permeability transition pore changes and Ca2+ overload results in MPTP activation.
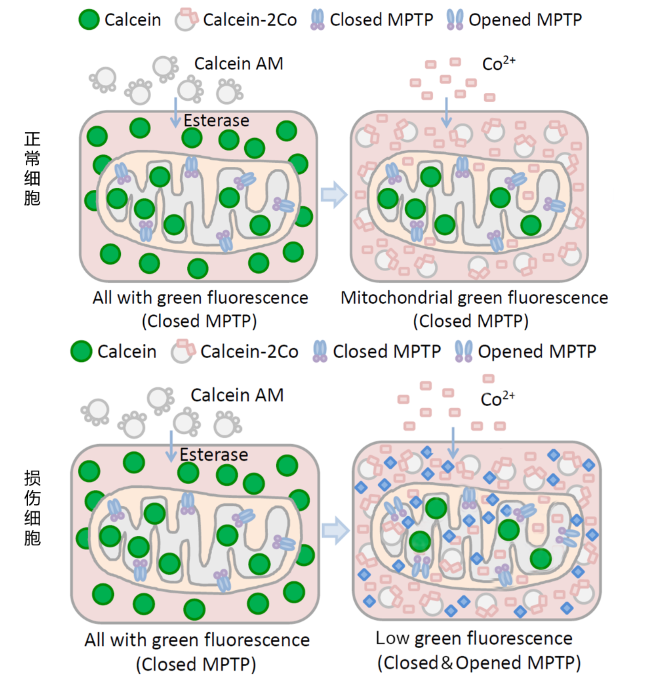
calcein acetoxymethyl ester (Calcein AM; A nonpolar dye that can fluorescently stain living cells. Calcein AM is passively transported into cells and accumulates in cytoplasmic components including mitochondria. Calcein AM can be hydrolyzed by esterase to remove acetyl methyl ester in cells and produce Calcein or Fluorexon, a polar fluorescent dye with no membrane permeability. Thus, Calcein is retained in cells and mitochondria, making cytoplasm including mitochondria show strong green fluorescence. CoCl2 was added to quench the green fluorescence of Calcein in the cytoplasm. Under normal conditions, MPTP of mitochondria is turned off and CoCl2 cannot enter mitochondria. Therefore, only Calcein in cytoplasm is quenched in normal cells. However, MPTP of the injured cells is open to a certain extent, at which CoCl2 can enter the mitochondria. Therefore, in addition to all Calcein in the cytoplasm, Calcein in mitochondria is also quenched to varying degrees in the injured cells. At this point, the green fluorescence signal of Calcein can be detected by flow cytometry, and the degree of MPTP openness can be determined by comparing the intensity of the fluorescence signal, thus inferring the degree of mitochondrial damage.
Frequently Asked Questions
Q: What if the fluorescence in the cytoplasm is not quenched after adding cobalt chloride
A: The concentration and dosage of Calcein-AM can be appropriately reduced.
Q: How should the final results be analyzed? How should different subgroups be compared
A: The degree of mitochondrial MPTP opening can be judged according to the strength of Calcein green fluorescence in mitochondria. The stronger the green fluorescence is, the lower the degree of opening is, and the weaker the green fluorescence is, the higher the degree of opening is.
Q: How does the kit measure MPTP openness?
A: Compared with the method based on mitochondrial membrane potential, this method can more directly detect the changes in the openness of mitochondrial permeability transition pore. The degree of mitochondrial MPTP opening can be determined according to the strength of Calcein green fluorescence in mitochondria. The stronger the green fluorescence, the lower the degree of openness, and the weaker the green fluorescence, the higher the degree of openness. Negative control and positive control need to be set for comparison in the experiment. Without sensitivity and detection limit data, fluorescence microscopy and flow cytometry can be detected.
Q: How to set the negative control and positive control of the kit?
A: Calcein AM staining solution was added to negative samples; Ionomycin control was added to the positive sample.
Q: Is it possible to dye the core with DAPI after dyeing the kit?
A: Abbkine's Hoechst 33342 Living cell dye (BMD0062) is recommended if nucleating. Not fixed.
Q: Which channel should the kit use for testing?
A: FITC/488 nm excitation light channel was used for detection.
Related product recommendation
| Name | NO. | Size |
| EdU Cell Proliferation Image Kit (Green Fluorescence)
|
KTA2030 | 100 T/500 T |
| One-step TUNEL Apoptosis Assay Kit (Green Fluorescence)
|
KTA2010 | 50 T/100 T |
| Annexin V-EGFP/PI Apoptosis Detection kit
|
KTA0005 | 20 T/50 T/100 T |
| Annexin V-AbFluor™ 488/PI Apoptosis Detection kit
|
KTA0002 | 50 T/100 T |
| Comet Assay Kit (3-Well Slides)
|
KTA3040 | 15 T/75 T |











