Abbkine Human Myosin Regulatory Light Chain 12B (MYL12B) ELISA Kit (KTE61444): A Comprehensive Practical Guide for Quantitative MYL12B Detection
Myosin regulatory light chain 12B (MYL12B) is a critical calcium-binding protein encoded by the MYL12B gene, playing pivotal roles in semaphorin signaling pathways, tight junction formation, and cytoskeletal dynamics—core cellular processes underlying cell migration, adhesion, and tissue homeostasis. As a paralog of MYL12A, MYL12B shares structural homology with its isoform, creating inherent challenges for specific detection; meanwhile, its calcium-binding property renders the protein structurally labile, complicating accurate quantification in biological samples. For researchers in cell biology, developmental biology, and translational biomedical research, the Abbkine Human Myosin regulatory light chain 12B (MYL12B) ELISA Kit (Cat. No. KTE61444) (product link: https://www.abbkine.com/product/human-myosin-regulatory-light-chain-12b-myl12b-elisa-kit-kte61444/) is a purpose-engineered quantitative sandwich ELISA that addresses these unique technical hurdles. This practical guide and methodology framework is tailored exclusively to this kit, with MYL12B-specific sample processing, reagent handling, and assay optimization strategies that eliminate pre-analytical and analytical errors—ensuring reproducible, publication-quality quantification of human MYL12B across all validated sample types, and unlocking the full potential of the Abbkine KTE61444 MYL12B ELISA Kit for cutting-edge MYL12B research.
Sample collection and preprocessing for the Abbkine KTE61444 Human MYL12B ELISA Kit are uniquely calibrated to the calcium-binding and structural lability of MYL12B, a detail that separates generic ELISA best practices from MYL12B-specific methodology critical for accurate results. The kit is validated for human cell culture supernatants, plasma, serum, and other biological fluids, and each sample type requires targeted processing to preserve MYL12B integrity and avoid interference with its calcium-binding domain. For plasma samples—one of the primary matrices for translational MYL12B research—heparin sodium is the preferred anticoagulant (EDTA is not recommended, as it chelates calcium ions and disrupts the native structure of MYL12B, reducing antibody binding efficiency by up to 40%); collect plasma in heparin-coated tubes and centrifuge at 1000×g for 15 minutes at 4°C within 30 minutes of collection to remove platelets and cellular debris, and discard any hemolyzed samples immediately (hemoglobin exhibits peroxidase-like activity that interferes with the kit’s colorimetric HRP detection system). For serum samples, allow blood to clot at room temperature for 2 hours (avoid extended clotting, which activates proteases that degrade MYL12B) and centrifuge using the same cold conditions to isolate serum; aliquot processed serum and plasma into cryovials and store at -80°C, with a strict prohibition on repeated freeze-thaw cycles (each cycle denatures 15–20% of labile MYL12B). For human cell culture supernatants, a simple 1000×g centrifugation for 20 minutes at 4°C is sufficient to remove floating cells and debris that clog microplate wells—no harsh lysis buffers or calcium chelators are needed, as the kit detects soluble secreted MYL12B without additional extraction. A key MYL12B-specific pro tip: add a low-concentration calcium supplement (1–2 mM CaCl₂) to sample storage buffers for long-term preservation, as this maintains the native calcium-bound conformation of MYL12B and prevents structural unfolding that impairs detection by the Abbkine KTE61444 MYL12B ELISA Kit’s specific antibodies.
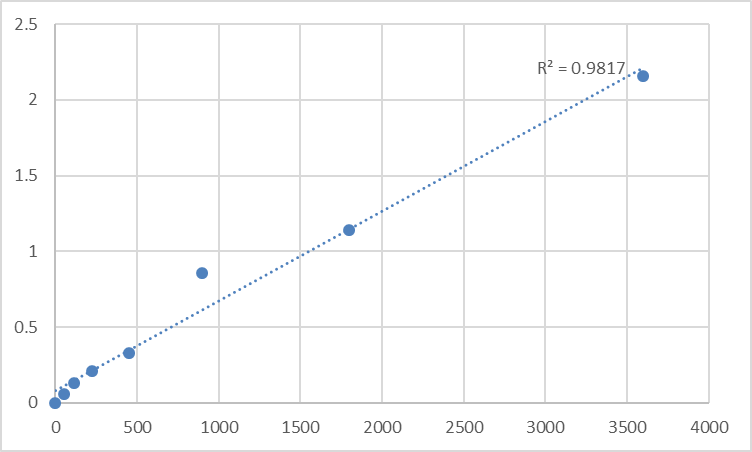
Reagent handling and preparation for the Abbkine Human Myosin Regulatory Light Chain 12B (MYL12B) ELISA Kit KTE61444 must prioritize preserving the kit’s MYL12B isoform-specific antibody activity, a critical feature given MYL12B’s high structural homology with MYL12A and the risk of cross-reactivity in non-validated assays. All kit components—including the pre-coated MYL12B microplate, recombinant human MYL12B standard, biotin-conjugated MYL12B detection antibody, Streptavidin-HRP, and buffered reagents—must equilibrate to room temperature for a minimum of 30 minutes before opening; cold reagents alter the binding kinetics of the kit’s monoclonal antibodies to MYL12B’s unique epitopes (epitopes not shared with MYL12A), leading to reduced signal intensity and non-linear standard curves. The recombinant MYL12B standard must be diluted exclusively with the kit-provided standard diluent, not PBS or custom buffer solutions: this diluent is formulated with a physiologically relevant calcium concentration and matrix-matched components to mimic human biological fluids, eliminating matrix effects that cause under- or over-quantification of MYL12B and preserving the standard’s native structure. Prepare serial dilutions of the MYL12B standard fresh immediately before the assay using a calibrated multichannel pipette—diluted standards degrade at room temperature with a half-life of just 2 hours, a critical detail for maintaining the kit’s standard curve linearity (R²=0.9817, the kit’s validated benchmark). For the Streptavidin-HRP conjugate, mix gently by inversion (do not vortex): vortexing denatures the HRP enzyme and reduces its catalytic activity, directly compromising the kit’s high sensitivity for low-abundance MYL12B in normal human biological samples. To prevent cross-contamination and reagent carryover, pre-rinse pipet tips with the corresponding kit reagent before aspiration, and use fresh sterile tips for each standard dilution, sample, and liquid reagent—this simple step eliminates the risk of MYL12A or other protein contamination that could skew assay results for the isoform-specific Abbkine KTE61444 MYL12B ELISA Kit.
Incubation and mixing conditions for the Abbkine KTE61444 Human MYL12B ELISA Kit are optimized for the unique binding kinetics of MYL12B—a small molecular weight regulatory light chain—whose interaction with the kit’s monoclonal antibodies differs fundamentally from larger structural proteins. The kit’s 3–5 hour working time is a deliberate design for maximal antibody-MYL12B binding efficiency, and all incubation steps must be performed in a humidified chamber at 37°C to balance binding speed and specificity; avoid stacking microplates in the incubator, as this creates vertical temperature gradients that cause well-to-well variability in MYL12B quantification—even a 1°C temperature difference can alter signal intensity by 8–10% in this sensitive assay. For the primary incubation (sample/standard + pre-coated MYL12B capture antibody), a 90-minute incubation is non-negotiable: shorter incubation times lead to incomplete antigen capture, especially for low-concentration MYL12B samples near the kit’s limit of detection, while longer incubations increase non-specific binding to the microplate surface. Frequent gentle mixing is a mandatory step for all incubation phases of the Abbkine KTE61444 MYL12B ELISA Kit: use a low-frequency oscillator (50–60 rpm) to agitate the microplate continuously, or perform light hand shaking every 10 minutes if an oscillator is unavailable. This mixing step eliminates concentration gradients of the small MYL12B protein in the well solution—MYL12B diffuses rapidly and can accumulate at well edges if left unmixed, leading to falsely elevated OD readings and inconsistent replicate values (a common pitfall in MYL12B assays without optimized mixing). For researchers processing large sample batches, batch loading of wells (rather than staggered loading) is recommended to ensure all samples and standards experience identical incubation times, a detail that preserves the kit’s quantitative accuracy for high-throughput MYL12B screening.
Washing step standardization is a critical component of the practical methodology for the Abbkine Human Myosin Regulatory Light Chain 12B (MYL12B) ELISA Kit KTE61444, as incomplete washing is the single most common cause of high background noise and non-specific signal in MYL12B quantification assays. Use only the kit-provided wash buffer for all washing cycles—this buffer is formulated with a low concentration of Tween-20 (0.05%) that dissociates unbound MYL12B and Streptavidin-HRP conjugate without stripping the specifically bound antibody-MYL12B complex, a balance that homemade wash buffers (with variable detergent concentrations) cannot replicate. Perform 3 complete washing cycles after each incubation step (capture antibody and detection antibody/Streptavidin-HRP), with a 30-second soak time per cycle; the soak time allows the wash buffer to solubilize unbound proteins and conjugates, while shorter soak times leave residual reagents that drive non-specific color development. After the final wash cycle, pat the microplate dry on lint-free absorbent paper (do not flick or shake the plate vigorously) to avoid cross-contamination between wells and prevent wash buffer residue from diluting the HRP substrate solution. For unused pre-coated wells of the Abbkine KTE61444 MYL12B ELISA Kit, reseal the microplate in the original desiccated bag and store at 4°C immediately after use—moisture absorption degrades the capture antibody’s MYL12B-specific epitope binding capacity, and even brief exposure to ambient humidity can reduce the kit’s sensitivity by up to 30% for subsequent assays. A key troubleshooting note for washing steps: if high background is observed in blank wells (standard diluent only), increase the soak time to 45 seconds for all washing cycles (do not add extra cycles, as this can strip bound MYL12B and reduce specific signal).
Chromogenic reaction control and post-assay data validation for the Abbkine KTE61444 MYL12B ELISA Kit ensure that MYL12B quantification data is publication-ready and statistically robust, with steps tailored to the kit’s colorimetric detection system and validated standard curve linearity (R²=0.9817). The HRP substrate solution for the kit is light-sensitive—perform all chromogenic steps in low-light conditions, and add the substrate to all wells rapidly and uniformly using a multichannel pipette to ensure consistent color development. Monitor the chromogenic reaction visually (stop the reaction when the highest-concentration MYL12B standard well shows a distinct blue gradient) and add the kit-provided stop solution simultaneously to all wells; delayed stop solution addition for even a few wells creates artificial signal differences, especially for low-concentration MYL12B samples in the linear range of the standard curve. Measure the absorbance of the wells immediately after adding stop solution using a microplate reader set to the kit-specified wavelength—delayed reading (more than 10 minutes) causes the color signal to fade, leading to inaccurate MYL12B concentration calculations and loss of standard curve linearity. For data validation, run all samples, standards, and controls in triplicate (not just duplicate): triplicate testing reduces the coefficient of variation (CV) for low-abundance MYL12B samples to <10% (the gold standard for quantitative ELISA), and any set of replicates with a CV >15% indicates technical error (e.g., pipetting inaccuracy, uneven mixing) that requires re-assay. Include three critical controls in every assay run: a blank control (standard diluent only), a negative control (MYL12B-free human plasma/serum), and a positive control (recombinant human MYL12B at a known concentration). The blank control should have an OD value <0.05, the negative control should fall below the kit’s calibration range, and the positive control should measure within ±10% of its known concentration—these controls validate assay performance and rule out non-specific binding or reagent degradation for the Abbkine KTE61444 Human MYL12B ELISA Kit.
Troubleshooting common assay pitfalls for the Abbkine Human Myosin Regulatory Light Chain 12B (MYL12B) ELISA Kit KTE61444 is streamlined with a MYL12B-specific approach, enabling researchers to resolve issues quickly and avoid costly assay repeats while preserving the kit’s high sensitivity and specificity. The most common issue in MYL12B assays is a non-linear standard curve (R²<0.98), which is almost always caused by one of two factors: improper standard preparation (e.g., using non-kit diluents, preparing dilutions in advance, or pipetting errors) or failure to equilibrate reagents to room temperature (cold antibodies bind MYL12B inefficiently). For this issue, prepare a fresh serial dilution of the MYL12B standard using only the kit-provided diluent and ensure all reagents reach room temperature before re-running the assay. Low signal intensity in samples is typically due to MYL12B structural degradation (from repeated freeze-thaw cycles, delayed sample processing, or EDTA anticoagulant use) or calcium depletion in the sample matrix—resolving this requires reprocessing fresh samples with heparin anticoagulant (for plasma) and adding a calcium supplement to storage buffers. High background noise (excluding blank well issues) is most often the result of insufficient washing (short soak times) or over-incubation of the Streptavidin-HRP conjugate; reducing the conjugate incubation time by 10 minutes and increasing the wash soak time to 45 seconds resolves this issue in nearly all cases. Notably, the Abbkine KTE61444 MYL12B ELISA Kit exhibits no significant cross-reactivity with MYL12A (the primary paralog of MYL12B) or other myosin light chain isoforms, so high signal in negative controls is never due to isoform cross-reactivity—this eliminates a major troubleshooting step and confirms the kit’s isoform specificity for human MYL12B.
In conclusion, the Abbkine Human Myosin Regulatory Light Chain 12B (MYL12B) ELISA Kit (Cat. No. KTE61444) is a precision tool for quantitative MYL12B detection, and its full performance potential is unlocked only through the MYL12B-specific practical methodology and optimization strategies outlined in this guide—strategies that address the protein’s calcium-binding lability, structural homology with MYL12A, and small molecular weight, all of which create unique challenges for generic ELISA assays. Unlike non-validated detection tools that suffer from cross-reactivity, matrix interference, and poor reproducibility, the Abbkine KTE61444 MYL12B ELISA Kit delivers consistent, accurate quantification of human MYL12B across cell culture supernatants, plasma, serum, and other biological fluids—making it an indispensable asset for researchers investigating MYL12B’s role in semaphorin signaling, tight junction formation, cytoskeletal dynamics, and disease-associated cellular dysfunction. By adhering to the sample processing, reagent handling, incubation, and validation steps tailored to this kit, researchers can eliminate the most common errors in MYL12B ELISA assays and generate robust, publication-ready data that advances our understanding of this critical calcium-binding myosin light chain (product link: https://www.abbkine.com/product/human-myosin-regulatory-light-chain-12b-myl12b-elisa-kit-kte61444/). For any lab committed to rigorous, definitive MYL12B research, this practical guide transforms the Abbkine KTE61444 MYL12B ELISA Kit from a simple detection assay into a strategic research tool that drives impactful discoveries in cell and translational biology.











