A decade of research on glycolysis: from energy factory to core of life regulation
The past decade has witnessed explosive breakthroughs in glycolysis research. Systematic progress has been made in subcellular localization, regulatory mechanisms, physiological and pathological functions, and clinical translation. These advancements not only reveal the complex regulatory network of this ancient metabolic pathway but also provide novel therapeutic targets and strategies for disease treatment and regenerative medicine. Below is a curated summary of the latest research highlights:
| dimension | traditional view | Breakthroughs in the last decade |
| 1. Location and organizational structure | The glycolysis is a soluble pathway which is evenly distributed in the cytoplasm. | Precise subcellular partitioning regulation forms efficient local energy supply system |
| 2. Core regulatory mechanisms | Glycolysis is a passive, substrate-driven metabolic pathway, which is mainly regulated by classical feedback mechanisms (e.g. ATP inhibition). | It is an active, multi-dimensional dynamic control network |
| 3. Core physiological functions | The core function is to generate ATP (energy supply) and provide a carbon skeleton (a precursor for biosynthesis). | Function far beyond energy supply, is the key cell fate decision hub |
| 4. Clinical translation perspective | The glycolysis is the background feature of disease, and there is no effective specific target. | Glycolysis is a promising therapeutic target, giving rise to a variety of new strategies |
Ⅰ. Disruptive Discovery of Subcellular Localization and Histological Form
The conventional view holds that glycolysis occurs exclusively in the cytoplasm, but studies over the past decade have demonstrated its precise subcellular compartmentalization, forming an efficient localized energy supply system:Cell Membrane/Cortex Self-Organizing Waves: A 2025 study published in *Nature Communications* by Johns Hopkins University researchers revealed that five core glycolytic enzymes—including aldolase and phosphofructokinase—form self-organizing propagating waves on human cell membranes/cortex. These waves co-localize with actin waves and concentrate locally, with enzyme concentrations reaching 3-10 times those in cytoplasm. This wave-like organization significantly boosts local glycolytic efficiency, generating ATP that accounts for 33% of total ATP production during glycolysis, providing immediate energy for cellular dynamics like migration and phagocytosis. Crucially, cancer cells with high metastatic potential exhibit markedly higher glycolytic wave activity and greater dependence on glycolysis compared to low-metastasis cells. This discovery offers a novel molecular mechanism for explaining the cancer Werber effect.
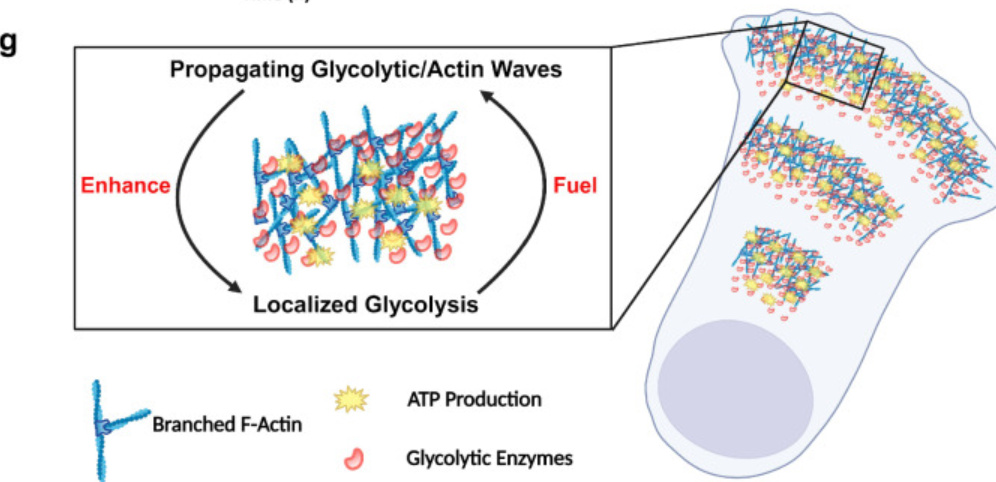
Figure g demonstrates that the interaction between glycolytic enzymes and traveling waves enhances localized glycolytic activity, thereby promoting the formation of additional waves.
DOI: https://doi.org/10.1038/s41467-025-60596-6
Organelle-targeted localization: Research has demonstrated that certain glycolytic enzymes can specifically target organelles such as mitochondria and the endoplasmic reticulum, forming "metabolic microdomains". For instance, the key glycolytic enzyme PGK1, after undergoing glycosylation modification, can translocate to mitochondria, directly regulating the coordinated switching between mitochondrial metabolism and glycolysis. This positional shift plays a pivotal role in the metabolic reprogramming of tumor cells.
Ⅱ. Deep Analysis of the Regulation Mechanism: Precise Regulation of Multi-dimensional Network
Recent studies have demonstrated that glycolysis is not a passive energy supply pathway, but rather a dynamic regulation mechanism involving multiple dimensions such as protein modification, cell cycle coupling, and mechanosensory perception.
1). The precise coupling between cell cycle and metabolism: The coordinated regulation mechanism of cell division and energy supply has been progressively elucidated.
A 2025 Nature study by the U.S. National Cancer Institute revealed that APC/C depends on mTOR's transient inactivation during the G0/G1 transition, enhancing glycolysis to provide energy for cells to transition into the proliferative phase. This discovery not only establishes a new link between cell cycle and metabolic regulation, but also offers fresh insights into how cells transition from quiescence to proliferation.
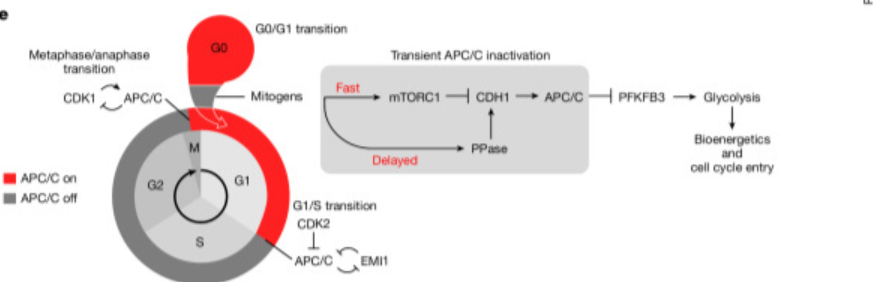
Figure e: The overall model of mTOR–APC/C coupling
DOI: 10.1038/s41586-025-09328-w
In 2020, a study by Liu Jiankang's team at Xi' an Jiaotong University published in *Cell Research* revealed that mammalian cells primarily utilize the TCA cycle during the G1 phase but prefer glycolysis during the S phase. This discovery uncovered the mechanistic link between two cancer markers: abnormal cell cycle and glycolytic addiction.
DOI: 10.1038/s41422-020-0372-z
2). Regulation of Protein Modification and Molecular Interaction
Glycosylation modification: In 2020, the Yi Wen research team at Zhejiang University reported in Nature Communications that O-GlcNAc glycosylation at the T255 site of PGK1 not only enhances its enzymatic activity but also induces mitochondrial translocation and inhibits the tricarboxylic acid (TCA) cycle, thereby promoting tumor growth.
DOI: 10.1038/s41467-019-13601-8
A 2024 study from Shandong University revealed that HK2-induced lactic acid influences gene expression through histone lactylation, playing a key role in the activation of hepatic stellate cells (HSC) and the progression of liver fibrosis, providing a potential new target for the treatment of liver fibrosis. DOI: 10.1016/j.cmet.2023.06.013
Protein interaction-mediated stability regulation: Research has identified DDX39B as an independent biomarker associated with poor survival outcomes in non-small cell lung cancer (NSCLC) patients. Through interaction with TRIM28, DDX39B undergoes TRIM28-mediated ubiquitination, which stabilizes and upregulates DDX39B, ultimately promoting cancer invasion and epithelial-mesenchymal transition (EMT) reprogramming. Furthermore, screening and validation experiments revealed that artemisinin, an FDA-approved drug, can target the DDX39B-TRIM28 interaction interface, demonstrating significant inhibitory effects on NSCLC metastasis and indicating its potential as a therapeutic agent.
DOI: 10.1038/s41392-025-02305-9
3). Mechanical signals and microenvironment sensing and regulation
A 2020 study by the University of Texas team, published in *Nature*, revealed for the first time how cytoskeletal-mediated mechanical signals regulate glycolysis. When cells transition from a rigid to a soft substrate, stress fiber disintegration triggers the release of E3 ubiquitin ligase TRIM21, which specifically degrades the glycolysis-limiting enzyme PFK, thereby suppressing glycolysis. Conversely, lung cancer cells can maintain high glycolytic rates by downregulating TRIM21 to adapt to the rigidity changes in the tumor microenvironment. This mechanism provides a novel perspective for understanding tumor metabolic plasticity.
DOI: 10.1073/pnas.2008801117
III. EXPANSION OF PHYSIOLOGICAL AND PATHOLOGICAL FUNCTIONS: FROM STEM CELL REGULATION TO IMMUNE TOLERANCE
The functions of glycolysis have gone far beyond energy supply, playing a key role in physiological processes such as stem cell fate determination, immune regulation, and disease development.
1). The "metabolic switch" of stem cell fate
A 2024 study published in *Cell Stem Cell* by the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences revealed that mitochondrial pyruvate transporter Mpc2 regulates airway basal stem cell function through glycolysis and epigenetic remodeling. Mpc2 knockout reduces intracellular citrate levels and acetyl-CoA synthesis, disrupting histone acetylation modifications and thereby inhibiting basal cell differentiation while maintaining stemness. Exogenous citrate supplementation restores differentiation efficiency in airway basal cells of chronic obstructive pulmonary disease (COPD) patients and promotes functional ciliated cell generation.
DOI: 10.1016/j.stem.2024.09.015
A research team at Huazhong University of Science and Technology developed a nano-composite hydrogel that activates bone marrow mesenchymal stem cells (BMSCs) by upregulating the HIF-1α/GLUT1 pathway, thereby enhancing osteogenic differentiation. Simultaneously, it suppresses excessive glycolysis in macrophages (M1 polarization) to reduce inflammation, resulting in a 30% faster callus formation in diabetic mice with fractures.
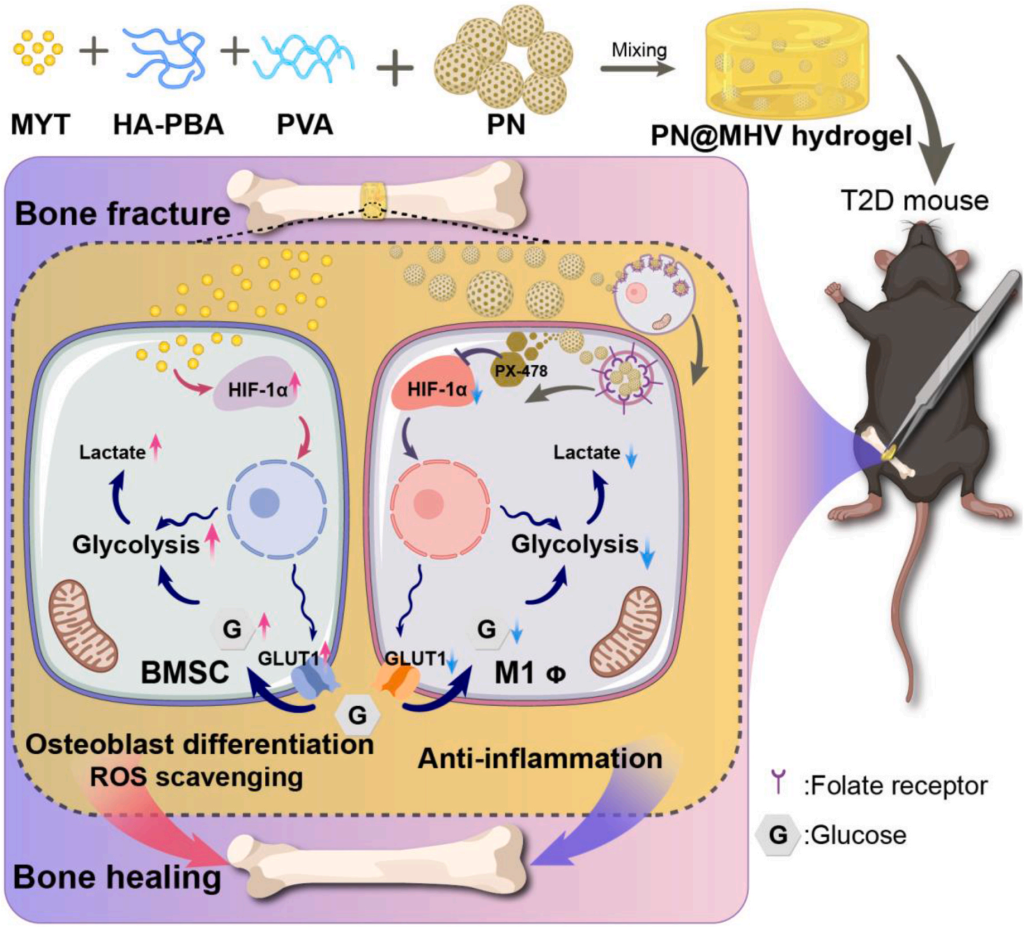
Schematic of preparation of PN@MHV hydrogel and promotion of T2D fracture healing by bidirectional regulation of glycolysis in BMSCs and macrophages
DOI: 10.1016/j.bioactmat.2025.03.020
Embryonic development studies demonstrate that glycolysis precisely guides stem cell differentiation into mesoderm or endoderm by activating signaling pathways such as Wnt and Nodal. Furthermore, regulating medium glucose concentration can directionally induce tissue differentiation, whereas blocking glycolysis results in embryonic tissue type imbalance.
2). Regulatory hubs of tumor immune tolerance
A 2023 study in *Cell Metabolism* by Tsinghua University School of Medicine revealed that aerobic glycolysis in tumor cells may enable immune evasion through specific mechanisms. Clinical data indicate that patients with low expression of glycolytic/Glut1 markers respond better to immune checkpoint blockade (ICB) therapy, providing a rationale for combination therapy.
DOI: 10.1016/j.cmet.2023.07.001
IV. Clinical Translation Progress: New Track of Targeted Therapy
Building on breakthroughs in glycolysis mechanisms, a series of targeted strategies have entered the phase of basic research or clinical exploration:
1). Cancer treatment targets: Targeting the self-organizing wave of glycolysis can suppress the energy supply of highly metastatic cancer cells; regulating the mTOR-APC/C axis can modulate cancer cell metabolism and proliferation; inhibitors targeting DDX39B-PKM2 binding sites or Glut1 (e.g., BAY-876) can significantly enhance tumor sensitivity to chemotherapy or immunotherapy; the specific glycosylation sites of HK2 and PGK1 also represent potential entry points for targeted cancer therapy.
2). Regenerative medicine applications: By regulating the glycolytic state of stem cells, the efficacy of stem cell therapies in diseases such as fracture healing and COPD can be optimized. For instance, exogenous citric acid supplementation has demonstrated clinical translation potential.
3). Diagnostic technology optimization: 18F-FDG PET imaging, based on the principle of high tumor glycolytic activity, has become a routine method for clinical tumor localization and diagnosis. Its sensitivity and specificity have been continuously optimized through metabolic mechanism studies.
In the future, with the deepening of research in interdisciplinary fields such as glycolysis, epigenetics, mechanical signaling, and immune microenvironment, as well as the precise development of targeted drugs, this ancient pathway will demonstrate greater value in precision medicine, regenerative medicine, and related fields.











