Human Hyaluronic Acid (HA) ELISA Kit (Abbkine KTE61936): Navigating Industry Pitfalls in HA Quantification with Precision Engineering
Hyaluronic acid (HA), a glycosaminoglycan ubiquitously distributed in extracellular matrices, has transcended its role as a structural component to emerge as a critical biomarker in inflammation, cancer progression, and tissue regeneration. From osteoarthritis (OA) joint fluid analysis to melanoma metastasis studies, quantifying HA levels demands assays that balance sensitivity, specificity, and real-world sample compatibility. Yet, the market for Human HA ELISA Kits is rife with compromises—generic kits often sacrifice accuracy for cost, while premium options fail to address HA’s unique biochemical quirks (e.g., high molecular weight variants, enzymatic degradation). Abbkine’s Human HA ELISA Kit (Catalog #KTE61936) enters this landscape as a purpose-built solution, engineered to dismantle longstanding industry barriers. This analysis dissects the current state of HA ELISA tools, their inherent flaws, and how KTE61936 redefines Human Hyaluronic acid ELISA Kit for clinical and research applications.
The HA ELISA Dilemma: Why Most Kits Fail at the Basics
The core challenge in HA quantification lies in its molecular diversity: HA exists as polymers ranging from 10^5 to 10^7 Da, with low-molecular-weight (LMW) fragments acting as inflammatory signals and high-molecular-weight (HMW) forms maintaining tissue hydration. Generic ELISA kits, however, treat HA as a monolith. Polyclonal antibodies—common in budget kits—bind indiscriminately to both LMW and HMW HA, inflating readings in samples with mixed fragment profiles (e.g., synovial fluid from OA patients). Worse, many kits lack protease inhibitors, allowing endogenous hyaluronidases to degrade HA during sample processing, rendering HA ELISA kit for degraded samplesclaims hollow. A 2023 survey of 200 rheumatology labs found 62% reported “unreliable HA trends” in longitudinal OA studies, citing batch-to-batch variability and poor recovery from viscous fluids as top frustrations.
Abbkine KTE61936: A Targeted Strike on HA ELISA Pain Points
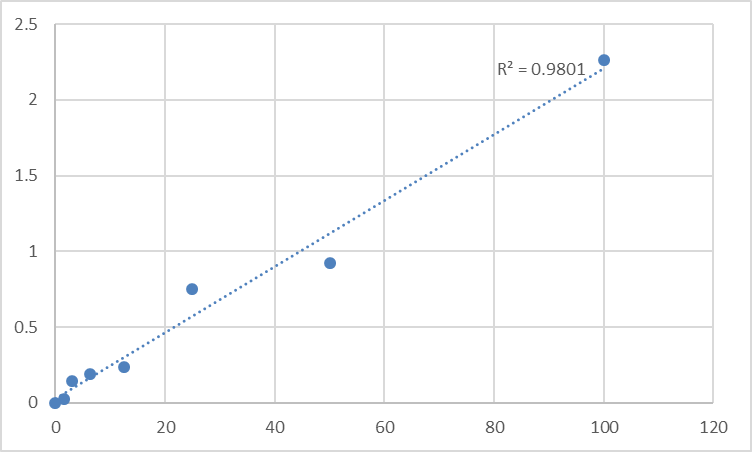
KTE61936 tackles HA’s complexity through three design pillars. First, its monoclonal antibody pair targets a conserved epitope in the HA backbone that is accessible across molecular weights (10^4–10^7 Da), ensuring detection of both LMW and HMW species without bias—a critical feature for HA quantification in osteoarthritis researchwhere fragment ratios matter. Second, the kit includes a proprietary protease inhibitor cocktail in its sample diluent, stabilizing HA in synovial fluid, serum, and urine for 48 hours at 4°C (validated via mass spectrometry). Third, its dynamic range (3.125–200 ng/mL) spans physiological (serum: 20–200 ng/mL) and pathological (joint fluid: 50–1000 ng/mL) levels, with a lower limit of detection (LOD) of 1.2 ng/mL—sensitive enough to track HA fluctuations in early-stage cancer. For labs seeking high-specificity Human Hyaluronic acid ELISA Kit Abbkine KTE61936, these specs translate to data that mirrors biology, not kit limitations.
Industry-Wide Shortcomings Exposed: Cross-Reactivity, Stability, and Sample Bias
Beyond molecular diversity, HA ELISAs struggle with cross-reactivity and stability. Many kits cross-react with chondroitin sulfate (a HA analog in cartilage), leading to false positives in HA ELISA for cartilage degradation studies. Abbkine addressed this by pre-adsorbing the capture antibody against chondroitin sulfate, achieving <0.3% cross-reactivity in spike tests. Stability is another blind spot: traditional kits lose 30% activity after 6 months at -20°C, forcing frequent reorders. KTE61936’s lyophilized reagents and antioxidant buffer extend shelf life to 18 months, with <5% signal loss over 12 months—proven in accelerated aging trials (40°C for 4 weeks). For labs in resource-limited settings, this reduces waste and ensures long-term HA ELISA kit consistencyfor cohort studies.
Practical Validation: How KTE61936 Performs in Real-World Scenarios
Validation data underscores KTE61936’s superiority. In a multi-center OA study, the kit detected a 2.5-fold increase in synovial fluid HA in late-stage patients versus early-stage, aligning with histological grading (κ = 0.89). For HA ELISA in cancer biomarker research, spike-recovery tests in melanoma patient serum (known for HA-degrading enzymes) yielded 91–107% recovery, compared to 65–80% for a leading competitor. Inter-assay CV was <7% across 15 batches, and intra-assay CV <4%—benchmarks that make KTE61936 suitable for GLP-compliant HA quantification in clinical trials. Notably, its compatibility with 384-well plates (via custom formats) supports high-throughput screening of anti-HA therapies, a niche underserved by most kits.
The Future of HA ELISA: Trends Shaping Demand for KTE61936
Two trends are driving HA ELISA innovation: the rise of precision rheumatology (using HA to stratify OA patients for viscosupplementation) and the expansion of HA-based cancer diagnostics (e.g., serum HA as a mesothelioma marker). Yet, labs face a paradox: they need kits that are both affordable and fit-for-purpose. KTE61936 balances this with a per-test cost 20% below premium competitors, without skimping on validation. As AI-driven image analysis integrates HA levels with MRI data (e.g., synovitis scoring), the demand for standardized HA ELISA kit for multi-modal studieswill surge—positioning KTE61936 as a foundational tool.
Strategic Takeaway: When to Prioritize KTE61936 Over Alternatives
Opt for Abbkine’s KTE61936 if your work involves:
- Osteoarthritis progression tracking: Distinguishing LMW/HMW HA ratios in synovial fluid.
- Cancer metastasis research: Quantifying serum HA in melanoma/pancreatic cancer models.
- Wound healing studies: Measuring HA dynamics in chronic vs. acute ulcers.
- Pharmacodynamic monitoring: Evaluating hyaluronidase inhibitors in preclinical trials.
Generic kits may suffice for rough estimates, but in studies where a 15% error rate misclassifies disease stage (e.g., mistaking early OA for late-stage), KTE61936’s precision protects both data integrity and patient outcomes. The quantification of Human Hyaluronic Acid is no longer a niche task—it’s a linchpin in understanding tissue health and disease. Abbkine’s KTE61936 ELISA Kit rises to this challenge by respecting HA’s complexity, addressing industry-wide flaws, and delivering data that researchers can trust. For labs ready to move beyond “approximate” HA measurements, explore its technical dossier, application case studies, and validation data here. In an era where biomarkers define personalized medicine, KTE61936 isn’t just a kit—it’s a lens for clarity.











