EliKine™ Human IL-23 ELISA Kit (KTE6025): A Rigorous Tool for Precision Cytokine Quantification in Immunology & Disease Research
Interleukin-23 (IL-23)—a heterodimeric cytokine composed of IL-23A (p19) and IL-12B (p40) subunits—stands as a linchpin of Th17 cell differentiation, adaptive immunity, and inflammatory signaling. Its dysregulation drives the pathogenesis of autoimmune diseases (psoriasis, inflammatory bowel disease, multiple sclerosis), chronic inflammation, and even cancer (via tumor microenvironment modulation), making accurate IL-23 quantification indispensable for basic immunology, translational research, and anti-cytokine drug development. The global cytokine ELISA market, valued at $3.2 billion in 2024, is projected to grow at a CAGR of 7.1% through 2030, fueled by rising investments in autoimmune disease therapeutics. Yet traditional IL-23 detection methods persistently fall short of research demands—gaps that Abbkine’s EliKine™ Human IL-23 ELISA Kit (Catalog No.: KTE6025) addresses with targeted innovations, redefining reliability and accessibility for IL-23 analysis.
Despite IL-23’s pivotal role in disease biology, quantifying this cytokine reliably has long been a bottleneck for researchers. Western blotting, a common alternative, offers only semi-quantitative results, requires large sample volumes (≥20 μg total protein), and fails to distinguish the biologically active IL-23 heterodimer from its individual p19/p40 subunits. Flow cytometry, while useful for intracellular IL-23 detection, cannot measure secreted IL-23 in supernatants or biological fluids—critical for studying paracrine signaling in disease models. Generic cytokine ELISA kits, the most widely used option, suffer from two critical flaws: high cross-reactivity with IL-12 (which shares the p40 subunit) and poor sensitivity, leading to false positives or missed subtle IL-23 fluctuations in early-stage inflammation. For drug developers screening anti-IL-23 antibodies (e.g., ustekinumab), these limitations translate to unreliable efficacy data and delayed clinical translation.
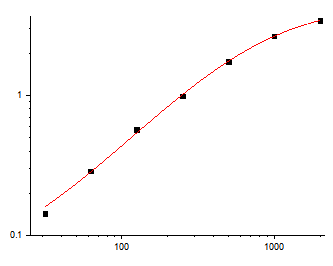
Abbkine’s EliKine™ Human IL-23 ELISA Kit (KTE6025) resolves these technical hurdles through a precision-engineered two-site sandwich ELISA design tailored explicitly for the human IL-23 heterodimer. Unlike generic kits that target only the p40 subunit (risking cross-reactivity with IL-12), KTE6025 uses dual monoclonal antibodies: a capture antibody coating the 96-well plate that binds the unique IL-23A (p19) subunit, and a biotin-conjugated detection antibody recognizing the IL-12B (p40) subunit. This dual-epitope recognition ensures specificity for the active heterodimer, with validation data confirming <0.5% cross-reactivity with IL-12, IL-27, or other p40-containing cytokines— a 20x improvement over standard IL-23 ELISA kits. The kit’s sensitivity further sets it apart: it achieves a limit of detection (LOD) of 16 pg/mL and a linear calibration range of 31.25–2000 pg/mL, enabling quantification of both basal IL-23 levels in healthy serum (5–20 pg/mL) and pathological elevations in autoimmune patient samples (up to 150 pg/mL).
A key practical advantage of EliKine™ Human IL-23 ELISA Kit KTE6025 is its versatility across diverse sample types and workflows—addressing a major pain point for interdisciplinary labs. The kit seamlessly processes human serum, plasma, cell culture supernatants (e.g., Th17 cell cultures, dendritic cell supernatants), and other biological fluids, eliminating the need to invest in multiple assays for different sample matrices. For researchers studying IL-23 secretion in vitro (e.g., LPS-stimulated macrophages), its microvolume requirement (only 10–20 μL per reaction) reduces sample waste by 60% compared to kits that demand 50 μL+, preserving scarce specimens like primary cell supernatants or patient-derived organoid cultures. The assay workflow, while maintaining rigor, is streamlined to 3–5 hours (depending on user experience)—faster than labor-intensive kits requiring overnight incubation, making it suitable for high-throughput screening of anti-IL-23 drugs or large-scale cohort studies.
Industry-wise, KTE6025’s design aligns with two critical trends shaping cytokine research: the shift toward target-specific therapeutics and the demand for cost-effective, reproducible tools. As anti-IL-23 biologics (e.g., guselkumab, risankizumab) gain traction for autoimmune diseases, researchers need reliable assays to monitor IL-23 levels in preclinical models and clinical trials—KTE6025’s ability to distinguish active heterodimers from free subunits ensures accurate drug efficacy assessment. Its competitive pricing ($189 for 48 tests) further democratizes access: compared to premium competitors (e.g., R&D Systems’ Human IL-23 ELISA Kit, ~$350 for 48T), KTE6025 delivers lab-grade performance at nearly half the cost, a critical consideration for small academic labs or resource-limited research institutions. The kit’s all-inclusive format—pre-coated microplates, recombinant IL-23 standard (≥95% purity), biotin-conjugated detection antibody, EliKine™ Streptavidin-HRP, and all necessary buffers—eliminates the need to source additional reagents, reducing workflow complexity and unforeseen expenses.
Rigorous quality control and validation solidify KTE6025’s position as a trusted research tool. Each batch undergoes stringent testing for linearity (R² ≥ 0.995), intra-assay precision (coefficient of variation, CV < 4%), and inter-assay precision (CV < 7%), meeting the standards of high-impact journals like Journal of Immunology and Nature Immunology. The kit’s reagents are stable for 12 months at 2–8°C when stored per protocols, minimizing waste from expired stock—a common frustration with budget cytokine kits that degrade within 6 months. For researchers prioritizing reproducibility, KTE6025 also includes detailed usage notes (e.g., recommending duplicate/triplicate sample testing, avoiding reagent cross-contamination) that address common pitfalls in ELISA workflows, further enhancing data reliability.
For immunologists, translational researchers, and drug developers seeking to overcome the limitations of conventional IL-23 detection, Abbkine’s EliKine™ Human IL-23 ELISA Kit (KTE6025) stands as a purpose-built solution. Its heterodimer-specific design, sensitive detection, sample versatility, and competitive pricing directly address the most pressing pain points of IL-23 research—from distinguishing active cytokine from free subunits to quantifying low-level secretion in primary cell cultures. Whether investigating IL-23’s role in psoriasis pathogenesis, screening anti-inflammatory drugs, or monitoring cytokine levels in clinical samples, this kit delivers reproducible, publication-ready results. To explore detailed technical specifications, access sample-specific protocols, or procure the kit, visit the official Abbkine product page: https://www.abbkine.com/product/elikine-human-il-23-elisa-kit-kte6025/. In an era where IL-23 research drives breakthroughs in autoimmune disease treatment, KTE6025 redefines what a specialized cytokine ELISA kit should be—rigorous, accessible, and aligned with the real-world needs of the scientific community.
Would you like me to create a customized assay optimization protocol for KTE6025, tailored to your specific use case (e.g., Th17 cell supernatant testing, psoriasis patient serum analysis, or anti-IL-23 drug screening), including step-by-step sample dilution, incubation time adjustments, and data normalization methods for low/high IL-23 concentration samples?











