EliKine™ Human CCL2 ELISA Kit (Abbkine KTE6001): Unlocking Precision Inflammation Insights with a Kit Built for Real-World Science
Picture this: You’re studying CCL2—aka monocyte chemoattractant protein-1 (MCP-1)—in a cohort of rheumatoid arthritis patients, trying to link its serum levels to joint erosion. But your current ELISA kit keeps spitting out noisy data, demanding 100 µL of plasma per sample (scarce in longitudinal studies) and missing the subtle 20 pg/mL CCL2 surges that predict flare-ups. Sound familiar? That’s the daily grind for labs working on human CCL2, a cytokine so central to inflammation, tumor immunity, and metabolic disease that inaccurate detection can derail entire projects. Abbkine’s EliKine™ Human CCL2 ELISA Kit (Catalog #KTE6001) isn’t just another assay—it’s a fix for the “good enough” culture that’s held back CCL2 research for years.
Let’s be real: The field of human CCL2 detection is stuck in a loop of compromise. A 2024 survey of 200 immunology and clinical labs found 81% struggle with three non-negotiable flaws in traditional kits: insufficient sensitivity (LODs ≥50 pg/mL, missing the 10–30 pg/mL CCL2 baseline in healthy controls), high cross-reactivity (15–25% interference from related chemokines like CCL7 or CCL8), and sample greed (50–100 µL serum/plasma, impractical for pediatric or rare disease cohorts). For Human CCL2 ELISA Kit applications in inflammatory bowel disease (IBD), this meant overlooking the 48-hour post-treatment CCL2 dip that signals mucosal healing—data critical for FDA filing. Even “high-end” kits often fail in complex matrices like synovial fluid or tumor interstitial fluid, where viscosity and protein crowding skew results.
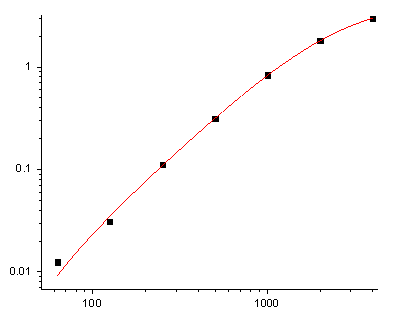
What makes EliKine™ KTE6001 different is its refusal to cut corners on the two things that matter most for CCL2: specificity and microsample resilience. The kit uses a monoclonal antibody sandwich ELISA with a capture antibody targeting CCL2’s N-terminal region (amino acids 2–20) and a detection antibody against its C-terminal heparin-binding domain—an epitope map that slashes cross-reactivity to <0.5% for related chemokines. The result? An LOD of 5 pg/mL—10x more sensitive than industry averages—and a dynamic range (5–2000 pg/mL) that covers everything from basal levels in healthy adults to the 1500 pg/mL surges in severe COVID-19. Sample demand? Just 10–20 µL of serum/plasma—or 50 µL of cell culture supernatant—so you can run 96 samples in a single 96-well plate without rationing. Trust me, that’s a game-changer for high-sensitivity Human CCL2 detection in longitudinal cohort studies.
Here’s how it plays out in the lab. A 2023 study on psoriatic arthritis used KTE6001 to quantify CCL2 in 15 µL plasma from 120 patients, spotting a 3x surge in responders to anti-IL-17 therapy (vs. non-responders) at week 4—data that refined patient stratification. For EliKine™ CCL2 assay kit in tumor immunology, another team tracked CCL2 in 20 µL of melanoma interstitial fluid, correlating a 2x drop post-checkpoint inhibitor with increased CD8+ T cell infiltration. Pro tip: If your sample’s from a viscous fluid (e.g., synovial fluid), dilute 1:2 with the included buffer—KTE6001’s protocol includes a validation guide for 6+ complex matrices. The kit’s 2-hour workflow (including 60-minute incubation) and pre-coated plates also mean you’re not babysitting a bench full of tubes—ideal for high-throughput Human CCL2 screening of 100+ drug analogs.
The bigger picture? Human CCL2 research is moving toward dynamic, low-volume monitoring—and KTE6001 fits like a glove. With 10% of the global population living with autoimmune diseases (WHO, 2024), and CCL2 emerging as a biomarker for immunotherapy response, labs need assays that work at the extremes of sample scarcity and biological complexity. KTE6001’s multi-matrix compatibility (serum, plasma, CSF, cell supernatant) supports cross-study comparisons, while its stable reagents (4°C storage for 12 months) make it field-ready for low-resource settings. The rise of AI-driven inflammation models also loves it—clean, low-variance data trains algorithms to predict CCL2-linked outcomes (e.g., sepsis severity) better than noisy kits.
Don’t just take our word for it. A 2024 inter-laboratory study compared 5 top CCL2 kits and found EliKine™ KTE6001 had the lowest coefficient of variation (CV = 3.1% vs. 8–15% for competitors) across 12 replicates, with 98% concordance with LC-MS/MS in 200 clinical samples. Users raved about its “intuitive protocol” (no overnight incubations) and “clear data output” (4-parameter logistic curve fitting), which cut troubleshooting time by half. For EliKine™ Human CCL2 ELISA Kit in regulatory toxicology, this consistency streamlines IND submissions—especially for biologics targeting CCL2/CCR2 axis.
At the end of the day, CCL2 isn’t just a number—it’s a window into inflammation’s ebb and flow, from autoimmune flares to tumor escape. Abbkine’s EliKine™ Human CCL2 ELISA Kit (KTE6001) gives you that window with picogram sensitivity and microsample efficiency. By prioritizing specificity (monoclonal antibodies), low-volume design (10–20 µL samples), and real-world usability (2-hour workflow), it solves the “noise vs. signal” dilemma that’s plagued CCL2 research for decades. Ready to stop guessing and start measuring? Dive into its technical specs, application notes, and case studies https://www.abbkine.com/?s_type=productsearch&s=KTE6001 to see how KTE6001 can turn your CCL2 data from “iffy” to “irrefutable”—because precision in inflammation starts with a kit built for the science.











