CheKine™ Micro Ethanol Content Detection Kit (Abbkine KTB3031): A Practical Guide to Precise Ethanol Quantification
Ethanol—whether as a biofuel, beverage component, clinical toxin, or industrial solvent—demands accurate quantification across sectors from biomanufacturing and food science to clinical diagnostics. Yet, common ethanol detection methods fall short: gas chromatography (GC) requires costly equipment and skilled operators, while generic enzyme assays suffer from matrix interference, and test strips deliver only semi-quantitative results. Abbkine’s CheKine™ Micro Ethanol Content Colorimetric Detection Kit (catalog KTB3031, available at https://www.abbkine.com/?s_type=productsearch&s=KTB3031) solves these pain points with a specificity-driven, user-friendly design. Priced at $79 for 48 tests/48 standards, this kit combines ethanol-selective enzyme chemistry with microplate compatibility, making precise ethanol measurement accessible to labs of all sizes. This practical guide delivers actionable strategies to master the kit—from sample-specific preparation to result validation—ensuring you unlock reliable, reproducible ethanol data for your application.
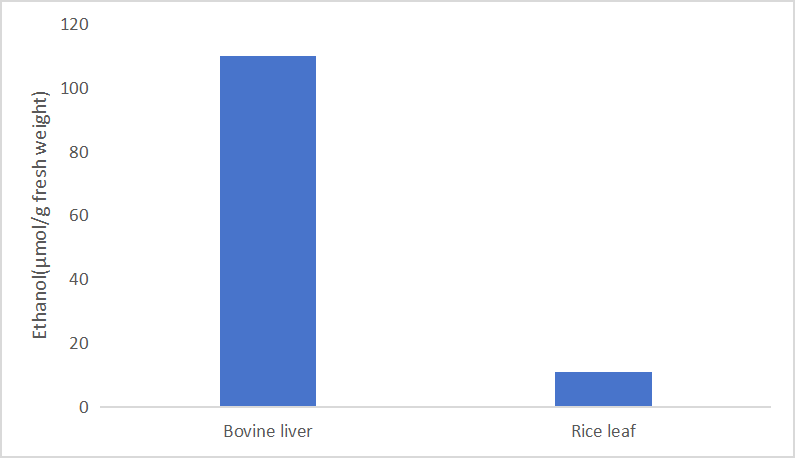
At the heart of CheKine™ Micro Ethanol Content Detection Kit KTB3031’s performance lies its ethanol-specific enzyme-coupled reaction. The kit leverages alcohol oxidase (AOx) to catalyze the oxidation of ethanol to acetaldehyde and hydrogen peroxide (H₂O₂), followed by a peroxidase (POD)-mediated reaction that converts a chromogenic substrate into a colored product. Unlike non-selective methods that react with other alcohols (e.g., methanol, isopropanol), AOx in KTB3031 exhibits strict specificity for ethanol, even in complex matrices. The chromogenic product’s absorbance at ~550nm correlates directly with ethanol concentration, with a detection range of 0.1–10 mg/mL—covering the needs of most applications (e.g., 0.5–5% ethanol in fermentation broths, 0.01–0.1% in clinical serum). A critical nuance: Abbkine’s buffer formulation includes a peroxide stabilizer to prevent H₂O₂ degradation, a common flaw in low-cost kits that leads to underestimated ethanol levels. For researchers and quality control teams, this specificity and stability translate to data you can trust—no more correcting for false positives or variable readings.
Sample preparation is make-or-break for ethanol quantification, and KTB3031’s protocol is tailored to mitigate matrix effects across diverse sample types. For fermentation broths (yeast, bacterial cultures): Centrifuge at 8,000×g for 5 minutes to remove cells/debris, then dilute 1:100 with the kit’s Sample Dilution Buffer to reduce sugar and protein interference. For clinical samples (serum, plasma): Dilute 1:50 to minimize hemoglobin and lipid interference—avoid hemolyzed samples, as red blood cell components inhibit AOx. For food/beverage samples (wine, beer, spirits): Dilute high-ethanol samples (e.g., 40% vodka) 1:1000 to fit the detection range; for carbonated drinks, degas by sonication for 5 minutes before dilution. For industrial solvents: Dilute 1:10000 to avoid overwhelming the enzyme system. A pro tip: For samples with high reducing sugars (e.g., fruit fermentation), add 1μL of glucose oxidase (100 U/mL) per 100μL sample to degrade glucose, which can cross-react with the chromogenic reagent.
Optimizing reaction conditions unlocks KTB3031’s full precision, and small adjustments can drastically improve data reliability. Start with temperature: Incubate at 37°C for mammalian-derived samples (clinical) or 30°C for microbial/fermentation samples—AOx activity peaks at these temperatures, ensuring complete ethanol oxidation. Incubation time: 30 minutes for samples with ethanol concentrations >1 mg/mL, 60 minutes for low-level samples (<0.5 mg/mL)—avoid over-incubation, as non-specific substrates may degrade over time. Reagent handling: Bring all components to room temperature (25°C) before use; cold reagents slow enzyme kinetics and reduce color development. Mixing is critical: After adding the Reaction Mix to samples, tap the microplate gently (don’t vortex) to avoid foam, which interferes with absorbance readings. For high-throughput runs, use a multichannel pipette to ensure uniform reagent volumes—even 1μL discrepancies can skew results in low-concentration samples.
Mitigating common interferences ensures that your ethanol readings reflect true concentrations, not matrix artifacts. The biggest culprits: methanol (in industrial samples), ascorbic acid (in fruit-based products), and proteins (in biological samples). For methanol-containing samples: The kit’s AOx has <5% cross-reactivity with methanol, but for samples with high methanol (e.g., biofuel blends), use the provided Methanol Inhibitor (included in select kit versions) to block methanol-AOx binding. For ascorbic acid: Add 0.1% ascorbate oxidase to the Sample Dilution Buffer to degrade ascorbic acid, which quenches the chromogenic product. For protein-rich samples: Include an additional centrifugation step (12,000×g for 10 minutes) to pellet proteins, or use the kit’s Protein Precipitation Reagent to clarify extracts. A quick validation: Run a “spiked recovery” test by adding a known amount of ethanol to your sample—recovery rates between 95–105% confirm no significant interference.
Converting raw absorbance data into meaningful ethanol concentrations requires rigorous standardization. First, construct a standard curve using the kit’s 48 pre-calibrated ethanol standards (0–10 mg/mL). Plot absorbance vs. ethanol concentration and fit with a linear regression (aim for R² ≥ 0.995)—this accounts for any minor variations in enzyme activity or substrate stability. Calculate sample ethanol concentration using the curve, then multiply by your dilution factor. For example, if a 1:100-diluted fermentation broth sample reads 0.5 mg/mL on the curve, the actual concentration is 0.5 × 100 = 50 mg/mL (5% v/v, assuming ethanol density = 0.789 g/mL). For clinical samples, express results as mg/dL (1 mg/mL = 10 mg/dL) to align with clinical reference ranges (e.g., legal blood alcohol concentration is ~80 mg/dL in most regions). Avoid the common mistake of using a single standard for calibration—linearity can vary across the detection range, so full curve generation is non-negotiable.
The versatility of CheKine™ Micro Ethanol Content Detection Kit KTB3031 aligns with the growing demand for multi-purpose ethanol assay tools across industries. In biomanufacturing, it monitors ethanol production in yeast fermentation for biofuel or recombinant protein expression—enabling real-time process optimization. In clinical diagnostics, it quantifies blood ethanol levels for intoxication screening or monitoring alcohol metabolism in patients with liver disease. In food science, it ensures compliance with beverage alcohol content regulations (e.g., 5% v/v for beer, 12% for wine) or tests for ethanol in non-alcoholic products. In environmental testing, it measures ethanol in industrial effluents to assess pollution levels. What sets KTB3031 apart is its ability to deliver lab-grade accuracy without the complexity of GC—at a cost-per-test of ~$1.65, it democratizes ethanol quantification for small breweries, academic labs, and clinical facilities alike.
Best practices for kit storage and handling extend its lifespan and maintain performance. Store all components at -20°C, and aliquot the AOx-POD Enzyme Mix into small volumes to avoid repeated freeze-thaw cycles—this preserves enzyme activity for up to 12 months. The Chromogenic Substrate is light-sensitive, so store it in amber tubes or wrap in aluminum foil. Once reconstituted, the Reaction Mix should be used within 2 hours to prevent substrate degradation. For long-term projects, track kit batches and include a positive control (known ethanol concentration) in every assay run—this identifies batch-to-batch variability early. With proper care, KTB3031 retains consistent performance, making it a cost-effective choice for ongoing ethanol detection needs.
In conclusion, Abbkine’s CheKine™ Micro Ethanol Content Detection Kit KTB3031 delivers the specificity, flexibility, and affordability required for precise ethanol quantification across clinical, industrial, and research applications. By following the practical strategies outlined—sample-specific preparation, optimized reaction conditions, interference mitigation, and rigorous standardization—you can consistently generate reliable data that drives decision-making. Whether you’re monitoring fermentation processes, screening for alcohol intoxication, or ensuring product compliance, this kit simplifies complex ethanol detection while maintaining scientific rigor. To integrate KTB3031 into your workflow, visit its product page at https://www.abbkine.com/?s_type=productsearch&s=KTB3031 and elevate your ethanol content analysis to publication-quality standards.
Would you like me to create a customized protocol template for your specific application (e.g., fermentation monitoring, clinical serum testing, food beverage quality control) to further streamline your experiments with KTB3031?











