Cell Metabolism | Subfamily Inhibitor Product Reveals Liver Acetyl-CoA Metabolism Mechanism! Provides New Strategy for Treatment of Neuroinflammation and Susceptibility to Depression
Research Title: Hepatic Acetyl-CoA Metabolism Regulates Neuroinflammation and Depression
Susceptibility Viaacetate
Journal: Cell Metabolism
Impact Factor: 30.9 Publication
Date: 2025
Author Team: Chen Jianguo's Team at Huazhong University of Science and Technology Collaborative
Product: KTA8010-Dual Luciferase Reporter Gene Detection Kit

Original link: https://pubmed.ncbi.nlm.nih.gov/40992374
I. Research Background
- Previous studies have predominantly focused on "abnormal brain energy metabolism and depression," yet the role of peripheral organs—particularly the metabolic hub, the liver—in regulating mood remains an unresolved mystery.
- In clinical practice, patients with abnormal liver function are often accompanied by neuropsychiatric symptoms, suggesting that the "liver-brain axis" may be involved in mood regulation, but the specific molecular mechanism is unknown.
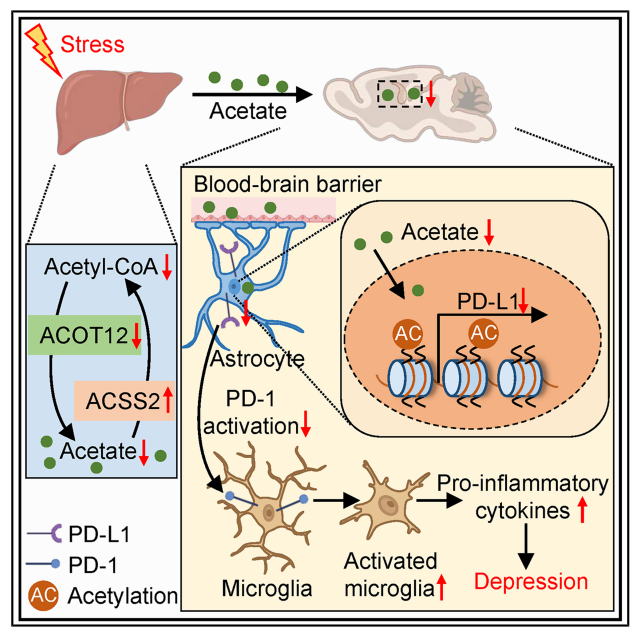
- Acetyl-CoA is a key metabolic intermediate, and the ACOT12 enzyme in the liver can hydrolyze it into acetate—a small molecule that crosses the blood-brain barrier but has never been shown to be linked to depression.
II. Research Objectives
The role of liver acetyl-CoA metabolism (particularly ACOT12 enzyme) in regulating brain neuroinflammation and depression susceptibility via acetate may fill the gap in the mechanism of 'liver-brain axis regulating mood'.
III. Research Approach
Ⅳ.research technique,research method
- Animal model: Chronic social defeat stress (CSDS) was used to construct depression model in mice, and the susceptible type (social avoidance and anhedonia) and resistant type mice were distinguished.
- Metabolic profiling: Analyze metabolites (pyruvate, acetyl-CoA, acetate, etc.) in the liver, kidneys, and colon of CSDS mice, with a focus on liver-specific changes.
- Molecular regulation: Using AAV virus to achieve "liver-specific overexpression of ACOT12" or "ACOT12 knockdown", we observed changes in depressive behaviors in mice and validated its effects by supplementing acetate.
- Brain region mechanism: Investigate the effects of acetate on brain regions (including the ventral hippocampus and prefrontal cortex), using RNA sequencing, immunofluorescence, and electrophysiological experiments to identify key molecules (PD-L1) and cells (astrocytes and microglia).
Ⅴ.Finding
1.Chronic stress triggers hepatic metabolic remodeling, with acetate serving as a key signaling molecule. Researchers employed a chronic social defeat stress model to simulate human depression and discovered that: in stress-sensitive mice, hepatic glucose metabolism was impaired, accompanied by significant reductions in acetyl-CoA and acetate levels. These changes were liver-specific, as acetate derived from gut microbiota remained unchanged. The hepatic energy metabolism disorder manifested as decreased ATP production and impaired mitochondrial function.
Conclusion: The liver underwent the metabolic conversion from glucose to acetate under stress, and acetate may be the "stress buffer signal" from liver to brain.

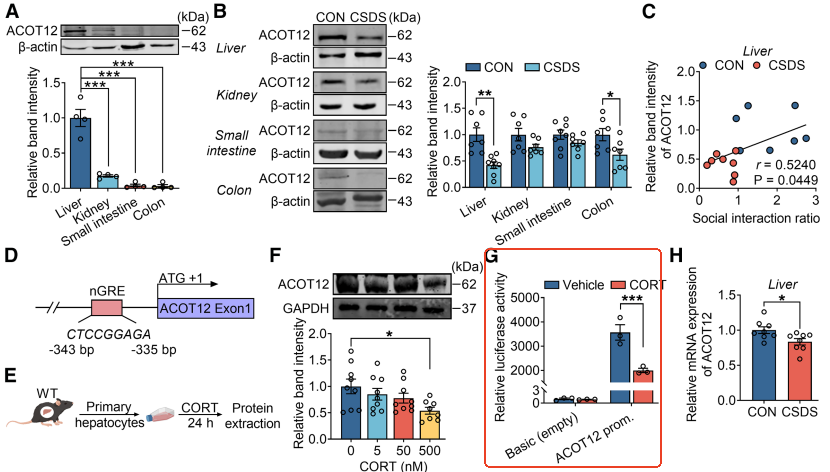
2.ACOT12, a key enzyme in hepatic acetate synthesis, is inhibited by stress hormones. Further studies revealed that ACOT12 serves as the primary acetyl-CoA hydrolase in the liver, with its expression markedly reduced in stress mice. Corticosterone, a stress hormone, directly inhibits ACOT12 transcription by binding to the negative glucocorticoid response element in its promoter region. Liver-specific overexpression of ACOT12 elevates blood acetate levels and alleviates depression-like behaviors. Conversely, ACOT12 knockdown renders mice more stress-sensitive, with even mild stress triggering depressive symptoms.
Conclusion: ACOT12 is a "switch molecule" that regulates the release of acetic acid in response to stress.
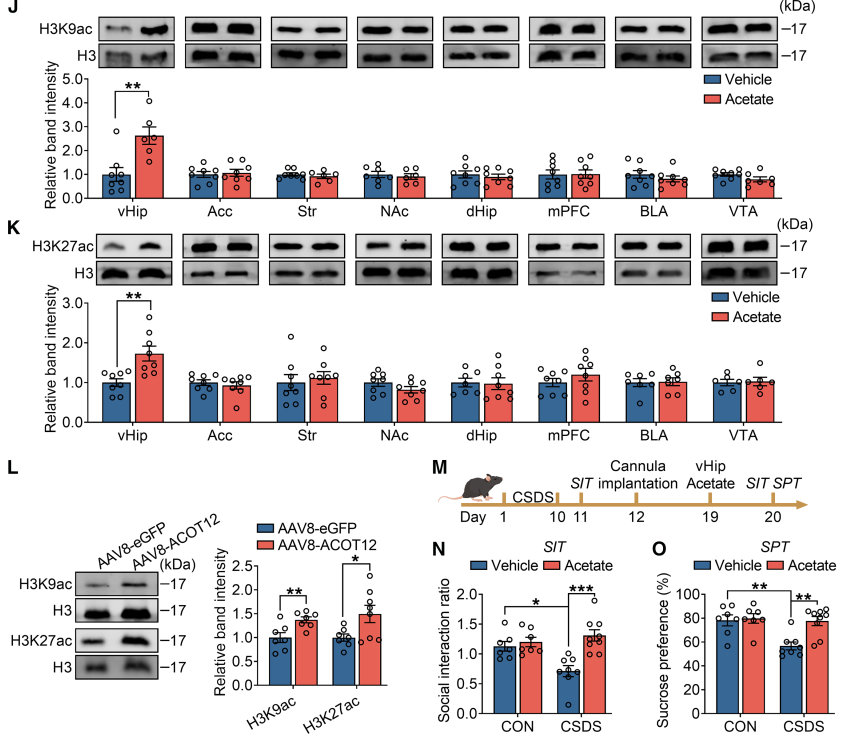
3.Acetate upregulates PD-L1 expression by enhancing histone acetylation in the ventral hippocampus. How does acetate act on the brain? Researchers found that exogenous acetate supplementation or liver overexpression of ACOT12 significantly increased histone H3K9ac and H3K27ac levels in the ventral hippocampus. These epigenetic modifications promote the expression of the immune checkpoint molecule PD-L1, which is primarily expressed in astrocytes, while its receptor PD-1 is expressed in microglia. Acetate is catalyzed by ACSS2 to convert into acetyl-CoA, thereby driving histone acetylation and gene expression.
Mechanism revealed: acetate → histone acetylation in ventral hippocampus → PD-L1 ↑ → inhibition of microglia activation → alleviation of neuroinflammation → improvement of depression behavior.
4.PD-L1 is the core effector molecule underlying the antidepressant effects of acetate. To further validate PD-L1's function: injecting recombinant PD-L1 protein into the ventral hippocampus mimics acetate's antidepressant effects; PD-L1 neutralizing antibodies block acetate's action; PD-L1 exerts neuroprotective effects by inhibiting microglia hyperactivation and restoring GABAergic synaptic function.
Significance: PD-L1 is not only a target for tumor immunotherapy but also a key molecule regulating emotional and immune balance in the central nervous system.












