Advancing Signal Transduction Research: Technical Evolution in PRKAR1B Protein Quantification
Within the intricate landscape of cellular signaling, the precise measurement of regulatory subunits like the Human CAMP-dependent protein kinase type I-beta regulatory subunit (PRKAR1B) is paramount. This protein plays a critical role in compartmentalizing the activity of Protein Kinase A (PKA), thereby fine-tuning cAMP-mediated responses involved in metabolism, gene expression, and neuronal signaling. Accurate quantification of PRKAR1B protein levels is therefore essential for dissecting molecular mechanisms in both physiological and pathological states, including metabolic disorders and neurological conditions. The demand for reliable, specific, and sensitive tools for the quantitative detection of PRKAR1B protein has driven significant methodological refinements in immunoassay technology.
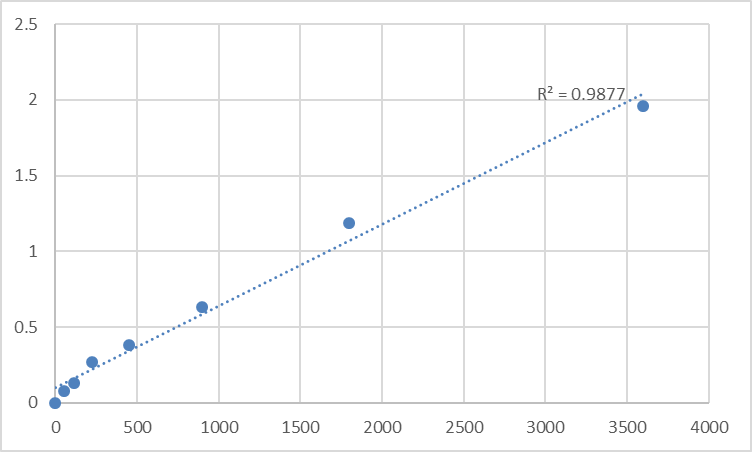
Historically, the analysis of low-abundance intracellular proteins such as PRKAR1B presented considerable challenges. Western blotting, while informative, often suffers from semi-quantitative results, limited throughput, and high sample consumption. Early-generation immunoassays frequently grappled with issues of cross-reactivity with homologous subunits like PRKAR1A, leading to compromised data specificity. The necessity to overcome these analytical bottlenecks catalyzed the development of more sophisticated, antibody-based detection platforms. The evolution towards highly optimized sandwich ELISA formats, exemplified by kits such as the Abbkine Human PRKAR1B ELISA Kit (KTE61151), marks a pivotal response to these enduring pain points in kinase research.
The technical trajectory of modern ELISA kits for targets like PRKAR1B emphasizes several key advancements. Contemporary designs incorporate pairings of highly specific monoclonal antibodies, drastically improving selectivity for the I-beta regulatory subunit over other isoforms. Enhanced assay sensitivity, often reaching the picogram per milliliter range, allows researchers to quantify PRKAR1B from limited biological samples, including serum, plasma, and tissue homogenates. Furthermore, the integration of streamlined protocols minimizes hands-on time and reduces procedural variability. The implementation of ready-to-use reagents and pre-coated plates, as seen in the referenced PRKAR1B sandwich ELISA kit, directly enhances reproducibility and throughput, enabling robust biomarker analysis and drug discovery screening.
A critical trend is the seamless integration of these detection kits into broader research workflows. The validated performance of a specific Human PRKAR1B immunoassay within complex matrices empowers scientists to correlate protein expression data with genetic or functional studies confidently. This compatibility is vital for exploring PRKAR1B's role as a potential biological marker for neurological function or metabolic research. The availability of such specialized kits accelerates hypothesis testing, moving beyond mere detection to precise, quantitative analysis that meets the stringent requirements of contemporary signal transduction studies and preclinical research.
Looking forward, the field of protein quantification will continue to converge with multiplexing and automation technologies. While dedicated single-analyte kits remain the gold standard for accuracy and sensitivity in quantifying specific targets like the cAMP-dependent protein kinase regulatory subunit, future iterations may incorporate digital or array-based platforms for parallel pathway analysis. The ongoing refinement of antibody specificity and assay dynamic range will further solidify the role of ELISA as an indispensable tool. Ultimately, the continued development and application of specialized reagents for PRKAR1B protein measurement are poised to unlock deeper insights into PKA regulatory biology, fostering advancements in understanding disease etiologies and identifying novel therapeutic intervention points.











