Abbkine’s DiO (DiOC18(3)) (Catalog BMD0072): A Practical Guide to Mastering Lipophilic Tracing with Green Fluorescent Precision
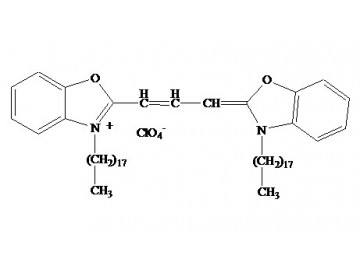
In the specialized field of cellular imaging and tracing, lipophilic carbocyanine dyes have become indispensable tools for visualizing membrane structures, tracking cell migration, and mapping tissue connectivity. Among these, DiO (DiOC18(3)) stands out for its bright green fluorescence, high membrane affinity, and minimal cytotoxicity—qualities that make it a top choice for researchers studying neural pathways, endothelial networks, and cell lineage dynamics. Yet, unlocking the full potential of DiO requires careful attention to sample preparation, dye handling, and imaging parameters—challenges that often lead to inconsistent results or suboptimal signal quality. This is where Abbkine’s DiO (DiOC18(3)) (catalog number BMD0072, available at https://www.abbkine.com/?s_type=productsearch&s=BMD0072) emerges as a reliable solution. Priced at $69 for 10mg and validated as a versatile lipophilic tracer, this reagent combines high purity, consistent performance, and accessibility to support diverse experimental needs. This practical guide delivers step-by-step optimization strategies, technical insights, and troubleshooting tips to help researchers maximize the utility of DiO (DiOC18(3)) BMD0072, ensuring reproducible, high-quality results in every tracing experiment.
Understanding the unique physicochemical properties of DiO (DiOC18(3)) is foundational to successful lipophilic tracing. As a lipophilic carbocyanine dye, DiO (DiOC18(3)) exhibits a long hydrocarbon chain (C18) that enables it to insert into lipid bilayers with high affinity, while its hydrophilic cyanine head group emits bright green fluorescence (excitation peak ~484nm, emission peak ~501nm) upon membrane binding. Unlike water-soluble dyes that require cell permeabilization, DiO (DiOC18(3)) passively diffuses into intact cell membranes, making it ideal for live-cell tracing and non-invasive imaging. Abbkine’s BMD0072 formulation prioritizes purity (≥95% via HPLC), eliminating impurities that can cause background fluorescence or reduce membrane binding efficiency. Key property to note: DiO (DiOC18(3)) is soluble in organic solvents (e.g., DMSO, ethanol) but nearly insoluble in aqueous buffers—this dictates critical steps in stock solution preparation and dilution. For researchers, this means avoiding direct addition of dry DiO to aqueous samples (which causes aggregation) and instead using organic solvents to create concentrated stocks, then diluting to working concentrations (typically 1–5 μg/ml) in culture media or buffer. Mastering this basic property prevents common pitfalls like uneven staining or weak fluorescence, setting the stage for successful experiments.
Optimizing stock solution preparation and storage is a critical first step to preserving the activity of DiO (DiOC18(3)) BMD0072. Start by preparing a 10mg/ml stock solution: dissolve the entire 10mg vial of BMD0072 in 1ml of anhydrous DMSO (preferred) or ethanol, vortexing gently to ensure complete dissolution. Avoid using aqueous DMSO (containing water) as it can induce dye aggregation over time. Aliquot the stock solution into small volumes (10–20 μl) in amber or foil-wrapped microcentrifuge tubes—this prevents light-induced degradation (DiO is photosensitive) and avoids repeated freeze-thaw cycles (which break down the dye’s hydrocarbon chain). Store aliquots at -20°C or -80°C for long-term stability (up to 12 months); avoid storing at 4°C, as this promotes dye precipitation. When ready to use, thaw an aliquot on ice (not at room temperature) and dilute to the desired working concentration in pre-warmed (37°C for live cells) culture media or imaging buffer. For example, to prepare 1ml of a 2 μg/ml working solution, add 0.2 μl of 10mg/ml stock to 999.8 μl of media. Key tip: Always mix the working solution thoroughly before application, as DiO can separate from aqueous media if not agitated—this ensures uniform staining across samples.
Tailoring staining protocols to specific experimental goals maximizes the utility of DiO (DiOC18(3)) BMD0072 across applications. For live-cell membrane labeling and migration tracking: Seed cells in imaging dishes or coverslips, allow them to adhere overnight (to 60–70% confluency), then add the working solution of BMD0072 directly to the culture media. Incubate at 37°C in a CO2 incubator for 15–30 minutes—longer incubation (up to 1 hour) may be needed for thick cell layers or tissues, but avoid over-incubation (which can lead to cytoplasmic leakage and background noise). After incubation, wash the cells 2–3 times with warm PBS to remove unbound dye—this step is critical for reducing background fluorescence. For fixed-cell or tissue staining: Fix samples with 4% paraformaldehyde (PFA) for 15 minutes at room temperature, then permeabilize with 0.1% Triton X-100 in PBS for 10 minutes (skip permeabilization if preserving membrane integrity is critical). Incubate the fixed sample with BMD0072 working solution (2–5 μg/ml) for 30 minutes at room temperature (protected from light), then wash 3 times with PBS. For neural tracing or tissue connectivity studies (e.g., mapping axonal projections): Apply a small volume (1–5 μl) of concentrated DiO stock (1–5mg/ml) directly to the target tissue region (e.g., brain slice, spinal cord explant) using a microinjector. Incubate the tissue in oxygenated buffer at 37°C for 24–72 hours to allow DiO to diffuse along lipid membranes—this passive diffusion enables visualization of entire cell networks without disrupting tissue structure.
Imaging optimization is key to capturing bright, specific signals with DiO (DiOC18(3)) BMD0072. Use a fluorescence microscope or confocal microscope equipped with a 488nm laser (or FITC filter set) for excitation and a 500–530nm emission filter—this matches DiO’s spectral properties and minimizes signal bleed-through from other fluorophores (e.g., DAPI, Texas Red) if multiplexing. For live-cell imaging, use a temperature-controlled stage (37°C) and CO2 chamber to maintain cell viability and prevent dye diffusion artifacts. Adjust exposure time (typically 100–500 ms) to avoid overexposure (which causes bleaching) while ensuring signal clarity—DiO’s bright fluorescence often allows shorter exposure times than less potent lipophilic dyes. For confocal imaging, use a pinhole size of 1 Airy unit to improve z-axis resolution, which is critical for visualizing membrane structures in 3D (e.g., dendritic spines, endothelial tubules). If experiencing weak fluorescence, increase the working concentration of BMD0072 (up to 5 μg/ml) or extend incubation time—avoid exceeding 10 μg/ml, as this can induce cytotoxicity in live cells. For multiplex experiments, combine BMD0072 with dyes emitting in non-overlapping spectra (e.g., DAPI for nuclei, Alexa Fluor 594 for proteins) to ensure distinct signal separation.
Troubleshooting common challenges with DiO (DiOC18(3)) BMD0072 ensures consistent, reliable results. If encountering dye aggregation (visible as bright, punctate spots in images): This typically stems from improper stock preparation or dilution. Remedy by re-preparing the stock solution with fresh anhydrous DMSO, vortexing thoroughly, and filtering the working solution through a 0.22 μm filter to remove aggregates. If background fluorescence is high: Increase the number of post-incubation washes (to 4–5) or reduce the working concentration of DiO—unbound dye is the primary source of background. For weak or uneven staining: Ensure the working solution is mixed thoroughly before application, and verify that the stock solution is not degraded (check for reduced fluorescence under a UV lamp). If staining is limited to cell surfaces (not diffusing into membranes): Extend the incubation time or increase the working concentration—this is common in cells with thick cell walls (e.g., plant cells) or high cholesterol content. For cytotoxicity in live cells: Reduce the working concentration (to 0.5–1 μg/ml) or shorten the incubation time—DiO is generally low-toxic, but high concentrations can disrupt membrane integrity over extended periods.
In the context of modern lipophilic tracing research, DiO (DiOC18(3)) BMD0072 addresses a critical industry need for affordable, high-performance tools that support diverse imaging workflows. As fields like neuroscience, developmental biology, and cancer research increasingly rely on visualizing cell connectivity and migration, the demand for lipophilic dyes that balance specificity, brightness, and low toxicity has grown. Abbkine’s BMD0072 meets this demand by offering a pure, consistent DiO formulation at a competitive price ($69/10mg), making it accessible to academic labs and industrial researchers alike. Its compatibility with live-cell, fixed-cell, and tissue imaging further enhances its utility, eliminating the need for multiple dyes for different applications. For researchers seeking to streamline their tracing experiments and achieve publishable results, BMD0072 is not just a reagent but a catalyst for efficient, reproducible research.
In conclusion, Abbkine’s DiO (DiOC18(3)) (catalog BMD0072) is a precision-engineered lipophilic tracer that simplifies and strengthens membrane imaging and cell tracing experiments. By following the practical guidelines for stock preparation, staining protocols, imaging optimization, and troubleshooting outlined in this guide, researchers can unlock the full potential of BMD0072—achieving bright, specific, and reproducible results that drive meaningful discoveries. Whether you’re tracking live cell migration, visualizing neural networks, or labeling cell membranes for multiplex imaging, BMD0072 delivers the performance and reliability that modern research demands. To integrate this essential lipophilic tracing tool into your workflow, visit its product page at https://www.abbkine.com/?s_type=productsearch&s=BMD0072 and take the first step toward more robust, insightful cellular imaging.











