Abbkine Human Phosphatidylinositol-Glycan Biosynthesis Class F Protein (PIGF) ELISA Kit (KTE61212): A Definitive Tool for GPI Anchor Biology and PNH Research
Phosphatidylinositol-glycan biosynthesis class F protein (PIGF) stands as a pivotal enzyme in the biosynthesis of glycosylphosphatidylinositol (GPI) anchors, a molecular machinery critical for tethering key proteins to the cellular surface membrane—and its dysregulation is the molecular cornerstone of paroxysmal nocturnal hemoglobinuria (PNH), a rare and complex acquired hematologic disorder characterized by hemolysis, recurrent infections, and venous thrombosis. For researchers in hematology, molecular cell biology, and rare disease drug development, the accurate and specific quantification of human PIGF is not just a research step, but a prerequisite for unraveling GPI anchor dysfunction mechanisms and advancing translational studies for PNH and related disorders. The Abbkine Human Phosphatidylinositol-glycan biosynthesis class F protein (PIGF) ELISA Kit (Cat. No. KTE61212) (product link: https://www.abbkine.com/product/human-phosphatidylinositol-glycan-biosynthesis-class-f-protein-pigf-elisa-kit-kte61212/) emerges as a gold-standard solution in this niche, addressing the unique challenges of human PIGF detection and delivering the reliable, reproducible data that defines impactful research in GPI anchor biology and PNH.
Engineered around the validated two-site sandwich ELISA platform, the Abbkine KTE61212 Human PIGF ELISA Kit is purpose-built to eliminate the specificity gaps that have plagued earlier PIGF detection tools in the research market. At its core, the kit features a microplate pre-coated with a highly specific monoclonal antibody targeting human PIGF, which selectively captures the target protein from complex biological samples; a biotin-conjugated secondary PIGF-specific antibody then binds to the captured antigen, with streptavidin-horseradish peroxidase (HRP) enabling signal amplification and colorimetric detection proportional to PIGF concentration. What sets this kit apart for GPI anchor research is its complete lack of significant cross-reactivity with PIGF analogues or other enzymes in the GPI biosynthesis pathway—a critical feature, as the GPI anchor assembly cascade includes multiple structurally related proteins that can confound non-specific detection assays. This stringent specificity ensures that measured PIGF levels reflect true in vivo or in vitro expression, rather than off-target signal from pathway homologs, a common source of experimental error in prior PIGF quantification efforts.
Sample versatility is a defining strength of the Abbkine Human Phosphatidylinositol-glycan biosynthesis class F protein (PIGF) ELISA Kit KTE61212, and it aligns perfectly with the diverse research needs of labs studying PNH and GPI anchor biology. Validated for use with human cell culture supernatants, plasma, serum, and other biological fluids, the kit eliminates the need for overly complex sample preprocessing protocols that risk PIGF degradation or loss—an important consideration for a protein whose expression is often analyzed in fragile hematologic samples from PNH patients. For hematology researchers working with clinical plasma or serum samples, this compatibility means direct quantification of PIGF from minimally processed patient material, preserving the integrity of the sample and enabling translational comparisons between in vitro cell models and human clinical specimens. Unlike specialized PIGF detection assays limited to a single sample type, this kit supports both basic laboratory research (e.g., measuring PIGF expression in GPI-deficient cell lines) and translational studies (e.g., profiling PIGF levels in PNH patient cohorts), creating a seamless bridge between bench and bedside—a key priority in rare disease research, where clinical sample access is often limited and precious.
The manufacturing and design of the Abbkine KTE61212 PIGF ELISA Kit prioritize experimental reproducibility, a non-negotiable requirement for research aiming to elucidate PNH pathogenesis or screen novel therapeutic candidates for GPI anchor disorders. The kit arrives as a fully integrated system, with all necessary components included and pre-optimized: pre-coated PIGF microplates, recombinant human PIGF standards, biotin-conjugated detection antibody, streptavidin-HRP, standard diluent, assay buffer, HRP substrate, stop solution, wash buffer, and plate covers. There is no need for researchers to source or prepare separate reagents, which eliminates the batch-to-batch variability that can arise from custom buffer preparation or mixed third-party components. The assay’s 3–5 hour working time is a deliberate balance of efficiency and accuracy, with incubation steps optimized for maximal antibody-antigen binding without the rushed kinetics that compromise data quality in so-called “rapid” ELISA kits. Additionally, the kit’s usage guidelines—including recommendations for duplicate or triplicate sample/standard testing, frequent gentle mixing during incubations, and fresh pipette tip use for all reagents—are tailored to minimize technical variability, ensuring that PIGF quantification data is consistent across experimental runs, lab members, and even different research facilities.
Storage and stability protocols for the Abbkine Human Phosphatidylinositol-glycan biosynthesis class F protein (PIGF) ELISA Kit are engineered to preserve the activity of its antibody components and standards, a critical detail for labs that may not run high-throughput PIGF assays on a continuous basis. The unopened kit is stable for long-term storage at 2–8°C, with no need for ultra-low temperature freezers that add cost and logistical complexity; unused pre-coated microplate wells can be stored desiccated at 4°C in the original sealed bag, maintaining the capture antibody’s binding affinity for future experiments. Shipping via gel pack with blue ice ensures that all components arrive at full activity, even for international research labs—a key consideration for the global rare disease research community, where PNH and GPI anchor disorder research is a collaborative effort across geographies. The kit’s clear storage instructions also mitigate the risk of reagent degradation, a common issue that can lead to reduced assay sensitivity and non-linear standard curves, ensuring that the kit delivers consistent performance from its first use to its expiration date.
The Abbkine KTE61212 Human PIGF ELISA Kit occupies a critical niche in the evolving landscape of rare disease research and GPI anchor biology, a field that has seen growing attention as targeted therapeutics for PNH and related disorders advance through clinical development. PNH is a rare hematologic disorder with a well-characterized molecular basis—defective GPI anchor biosynthesis due to PIGF and other pathway enzyme dysfunction—and the demand for reliable PIGF quantification tools has surged as researchers seek to develop therapies that restore GPI anchor function or target the downstream consequences of its loss. This kit serves as both a foundational research tool for elucidating the role of PIGF in GPI anchor assembly and a preclinical development tool for screening novel compounds: it enables the precise measurement of PIGF expression in cell models treated with candidate therapeutics, and the assessment of PIGF-mediated GPI anchor recovery in preclinical systems. As the biopharmaceutical industry increases investment in rare disease drug development, the need for validated, human-specific PIGF detection assays will only grow, and the Abbkine KTE61212 kit is uniquely positioned to meet this demand with its specificity, sample versatility, and reproducibility.
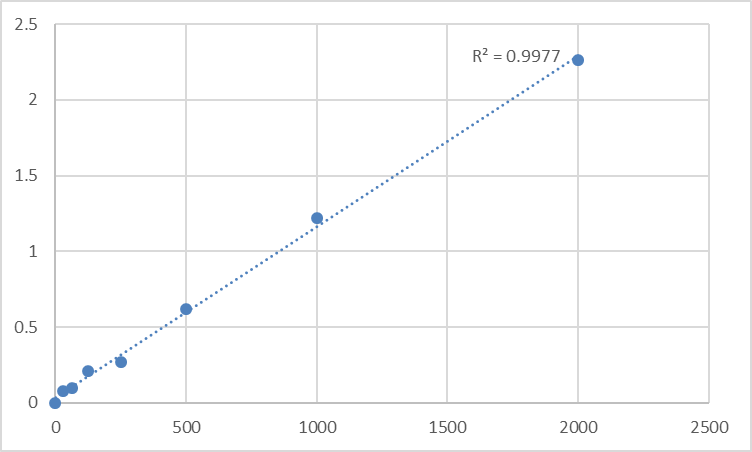
Adherence to rigorous research use guidelines and quality control standards further cements the Abbkine Human Phosphatidylinositol-glycan biosynthesis class F protein (PIGF) ELISA Kit KTE61212 as a trusted tool for the scientific community. Manufactured for research use only, the kit aligns with global regulatory standards for preclinical research tools, and its clear usage precautions—including prohibitions on mixing components from different kit lots and using expired reagents—ensure that experimental data meets the quality standards required for publication in high-impact hematology and molecular biology journals. The kit’s standard curve, optimized for human PIGF quantification, delivers high linearity, a hallmark of accurate ELISA performance, and the recommended experimental practices (e.g., room temperature reagent equilibration, pre-rinsing pipette tips) are designed to maximize the kit’s high sensitivity, even for low-abundance PIGF expression in GPI-deficient cell lines or PNH patient samples where the protein is dysregulated. For researchers, this means that data generated with the kit is not just reliable, but also defensible—an essential attribute for research that may inform future clinical trials for PNH and other GPI anchor disorders.
In the rapidly advancing field of GPI anchor biology and PNH research, the Abbkine Human Phosphatidylinositol-glycan biosynthesis class F protein (PIGF) ELISA Kit (Cat. No. KTE61212) stands as an indispensable tool, addressing the unique technical challenges of human PIGF quantification and delivering the specificity, versatility, and reproducibility that modern research demands. Its design aligns with the needs of both basic science labs unraveling the molecular mechanisms of GPI anchor biosynthesis and translational research teams advancing novel therapeutics for PNH, a rare disease with unmet clinical needs. As research into PIGF and GPI anchor dysfunction continues to expand, this kit will remain a cornerstone for accurate, human-specific PIGF detection, enabling discoveries that bridge molecular cell biology and clinical hematology (product link: https://www.abbkine.com/product/human-phosphatidylinositol-glycan-biosynthesis-class-f-protein-pigf-elisa-kit-kte61212/). For any lab committed to rigorous research in GPI anchor biology, PNH, or rare hematologic disorders, the Abbkine KTE61212 PIGF ELISA Kit is not just a detection assay—it is a strategic investment in high-quality, impactful research that has the potential to advance our understanding of these complex diseases and the development of life-changing therapies.











