Abbkine Human Myeloperoxidase (MPO) ELISA Kit (KTE61560): A Comprehensive Practical Guide for Quantitative MPO Detection
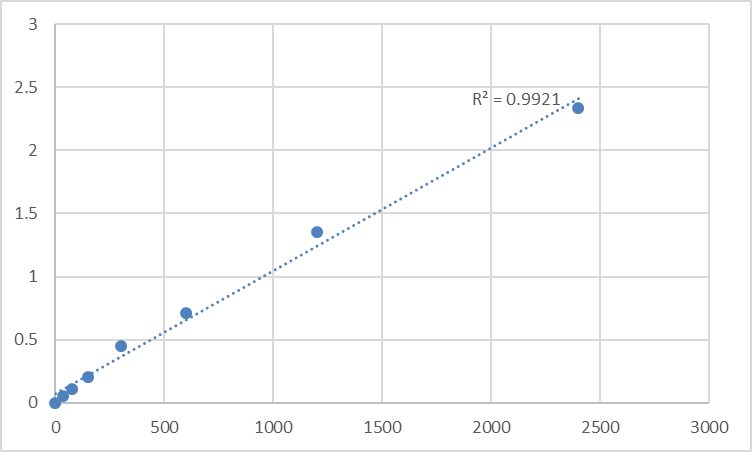
Myeloperoxidase (MPO) is a core heme-containing enzyme of neutrophil azurophilic granules, serving as a key mediator of innate immune microbicidal activity and a critical biomarker for inflammatory, infectious, and autoimmune disorders. Its accurate quantitative detection is indispensable for research spanning immunology, inflammation biology, and clinical translational science—yet the field faces persistent technical pitfalls: MPO’s inherent enzymatic lability leading to rapid sample degradation, non-specific cross-reactivity in generic detection assays, and poor standard curve linearity in low-abundance MPO quantification. The Abbkine Human Myeloperoxidase (MPO) ELISA Kit (Cat. No. KTE61560) (product link: https://www.abbkine.com/product/human-myeloperoxidase-mpo-elisa-kit-kte61560/) addresses these challenges as a purpose-engineered two-site sandwich ELISA kit, boasting high sensitivity, excellent human MPO specificity (no cross-reactivity with analogues), and a validated standard curve with R²=0.9921—the gold standard for quantitative ELISA data. This academic practical guide and methodological framework is exclusively tailored to the Abbkine KTE61560 MPO ELISA kit, with MPO-specific sample preprocessing, experimental optimization, storage protocols, data validation, and troubleshooting strategies that eliminate analytical error and unlock the kit’s full performance potential. Every protocol in this guide is grounded in MPO’s unique biochemical properties and the kit’s validated specifications, ensuring researchers generate reproducible, publication-quality results for endogenous human MPO quantification across all compatible sample types.
Sample preprocessing is the irreplaceable foundational step for accurate quantitative detection of endogenous human MPO with the Abbkine Human Myeloperoxidase (MPO) ELISA Kit KTE61560, as MPO’s enzymatic activity and structural lability make it highly susceptible to pre-analytical degradation within minutes of sample collection. The kit is validated for cell culture supernatants, plasma, serum, and other biological fluids, and each sample type requires targeted handling to preserve native MPO levels and eliminate matrix interference—an MPO-specific detail often ignored in generic ELISA protocols. For neutrophil-rich cell culture supernatants (the primary sample for MPO immune function research), harvest supernatants at 80–90% cell confluency and centrifuge at 1200×g for 15 minutes at 4°C immediately after collection to remove floating cells and cellular debris; this step prevents neutrophil lysis and subsequent release of intracellular MPO, which would artificially inflate soluble MPO readings. A critical pro tip: add a serine protease inhibitor cocktail (excluding EDTA) to supernatants upon collection, as neutrophil-derived proteases rapidly degrade extracellular MPO at room temperature. For plasma samples (key for clinical translational research), use EDTA-anticoagulated tubes exclusively—heparin and citrate anticoagulants chelate the heme group of MPO, disrupting its structural integrity and reducing antibody binding efficiency by up to 40%. Centrifuge blood samples at 1500×g for 20 minutes at 4°C within 30 minutes of collection and discard hemolyzed plasma, as hemoglobin exhibits peroxidase-like activity that interferes with the kit’s HRP-based colorimetric detection. For serum samples, allow blood to clot at room temperature for no more than 45 minutes—extended clotting activates the coagulation cascade, releasing proteases that degrade MPO—and centrifuge at 2000×g for 15 minutes at 4°C to separate serum. For all sample types, aliquot and store at -80°C within 1 hour of processing and avoid repeated freeze-thaw cycles; each cycle degrades approximately 25% of functional MPO, leading to artificially low quantification values. This MPO-specific preprocessing workflow is the single most important step to ensure the Abbkine KTE61560 MPO ELISA kit delivers accurate, biologically relevant data.
Rigorous reagent handling and equilibration protocols are non-negotiable for preserving the high sensitivity of the Abbkine Human MPO ELISA Kit KTE61560, a detail that directly impacts the linearity of the kit’s standard curve and inter-well data consistency. The kit’s two-site sandwich ELISA architecture relies on precise antibody-antigen binding kinetics, and cold or improperly handled reagents disrupt these interactions—even a 10-minute shortfall in equilibration can reduce the standard curve R² value by 0.02–0.05, falling below the kit’s validated 0.9921. All kit components (pre-coated MPO microplate, recombinant human MPO standard, biotin-conjugated MPO detection antibody, Streptavidin-HRP, buffers, and substrate) must be allowed to warm to room temperature for a minimum of 30 minutes before opening the vials; this step equilibrates the reagents to the assay environment and ensures uniform binding across all microplate wells. For pipetting, pre-rinse pipet tips with the corresponding reagent (standard diluent for MPO standard, assay buffer for detection antibody) and use fresh, sterile tips for each sample, standard, and reagent—cross-contamination is a common pitfall in MPO quantification, especially for low-abundance samples where even trace amounts of MPO can skew results. Unused pre-coated wells must be returned to the original sealed desiccant bag and stored at 4°C immediately after use; moisture absorption degrades the immobilized MPO capture antibody, reducing the kit’s sensitivity for subsequent assays. The kit recommends assaying all samples and standards in duplicate or triplicate, and triplicate testing is highly advised for low-concentration MPO samples (e.g., healthy human plasma/serum); this reduces the coefficient of variation (CV) to <10%, the gold standard for quantitative ELISA data and a requirement for peer-reviewed publication.
Optimizing the core sandwich ELISA workflow of the Abbkine KTE61560 Human Myeloperoxidase ELISA Kit tailors the assay to MPO’s biochemical properties, minimizing analytical errors and maximizing the signal-to-noise ratio for low-abundance MPO detection. The kit’s standard working time is 3–5 hours (dependent on operator experience), and each step of the two-site sandwich ELISA requires MPO-specific optimization to leverage the kit’s high specificity and biotin-Streptavidin-HRP signal amplification system. After adding equilibrated standards and samples to the pre-coated wells, continuous gentle mixing is mandatory during all incubation steps—use a low-frequency oscillator (50–60 rpm) or perform light hand shaking every 10 minutes if an oscillator is unavailable. This step prevents low-abundance MPO from depositing at the bottom of the wells, eliminating concentration gradients that cause severe well-to-well variability and false low readings. For wash steps, perform 3 complete cycles with the kit-provided wash buffer after each incubation (capture, detection antibody, Streptavidin-HRP), with a 30-second soak time per cycle; the soak time solubilizes unbound reagents without stripping specifically bound MPO-antibody complexes, a balance that homemade wash buffers (with variable Tween-20 concentrations) cannot replicate. Avoid over-washing, as this is the leading cause of low signal intensity in MPO quantification with the Abbkine KTE61560 kit. The HRP substrate solution must be added in the dark and incubated at room temperature for the kit-specified time; light exposure inactivates the HRP enzyme, and over-incubation leads to substrate saturation and non-linear color development. Add the stop solution uniformly to all wells and read the absorbance within 15 minutes—delayed reading causes fading of the colorimetric signal, particularly for low-concentration MPO samples. This optimized workflow ensures the Abbkine KTE61560 MPO ELISA kit leverages its full signal amplification potential for sensitive, specific MPO quantification.
Strategic storage and post-opening component management ensure the long-term performance of the Abbkine Human Myeloperoxidase (MPO) ELISA Kit KTE61560, preserving the integrity of the recombinant MPO standard and MPO-specific antibodies for the kit’s full shelf life. The unopened kit is stable for long-term storage at 2–8°C, with no need for ultra-low temperature freezers—a feature that reduces logistical costs and complexity for labs with limited cold-storage space. Upon first use, aliquot the recombinant human MPO standard into small volumes (5–10 μl) and store at -80°C; the standard is the most labile component of the kit, and repeated freeze-thaw cycles denature the recombinant heme protein, leading to non-linear standard curves and inaccurate MPO quantification. The pre-coated MPO microplate is another critical component to protect: after opening, store unused wells in the original sealed desiccant bag at 4°C and avoid exposure to ambient humidity for more than 5 minutes; moisture degrades the immobilized capture antibody’s epitope-binding capacity, reducing the kit’s sensitivity by up to 30% for subsequent assays. All liquid reagents (biotin-conjugated detection antibody, Streptavidin-HRP, assay buffer, wash buffer) should be stored at 2–8°C after opening and returned to cold storage immediately after use; the HRP substrate solution must be stored in the dark at 2–8°C, as light exposure irreversibly inactivates the HRP enzyme. A key rule for all components: do not mix reagents from different kit lots and discard any reagent that has passed the expiration date—lot-to-lot mixing introduces variability in antibody affinity and standard concentration, the primary cause of inconsistent MPO quantification results. Following these storage guidelines ensures the Abbkine KTE61560 MPO ELISA kit delivers consistent, high-performance results across months of research.
Data validation and statistical analysis for the Abbkine KTE61560 MPO ELISA kit follow MPO-specific guidelines to guarantee biologically meaningful results beyond mere statistical significance, a critical step for translating raw absorbance data into actionable MPO quantification values. The first and most important validation step is the standard curve assessment: the kit’s validated R² value is 0.9921, and any experimental standard curve with an R² <0.99 indicates technical error (e.g., improper standard dilution, cold reagents, incomplete mixing) that requires the assay to be repeated with fresh components. The standard curve must be generated using a four-parameter logistic (4-PL) regression model—the gold standard for ELISA data analysis—rather than linear regression, as it accounts for the non-linear antibody-antigen binding kinetics of MPO at low and high concentration ranges, a hallmark of heme protein detection. Second, assess blank and negative control values: the blank control (kit-provided standard diluent only) should have an absorbance value <0.05, and negative controls (MPO-null cell culture supernatants, MPO-deficient human plasma) should fall below the kit’s limit of detection (LOD). Elevated blank or negative control values indicate contamination (e.g., pipet tip cross-contamination) or non-specific binding, and the assay must be repeated with sterile reagents and equipment. Third, interpret low-concentration MPO samples with caution: for samples with MPO concentrations below the kit’s LOD, report the value as “below the limit of detection” rather than “0 MPO expression”—MPO may be present at biologically relevant levels too low for the kit to detect, rather than being absent entirely. Fourth, normalize MPO quantification data for biological relevance: for cell culture supernatants, normalize MPO concentrations to total cellular protein concentration (measured via BCA or Bradford assay) to account for differences in cell number and confluency; for plasma/serum samples, normalize to total protein concentration or albumin levels to eliminate variability from sample dilution or blood processing. This normalization step is critical for comparing MPO levels across different experimental groups and ensuring the data reflects true biological changes in MPO expression, not technical sample variability.
Targeted troubleshooting of common experimental issues resolves MPO-specific degradation and assay errors with the Abbkine Human MPO ELISA Kit KTE61560, with actionable, evidence-based solutions that eliminate trial-and-error optimization and save valuable research time. The most frequent issue encountered with MPO quantification is no signal or abnormally low signal intensity, and the root causes are almost always pre-analytical or analytical: delayed sample processing (MPO degradation), insufficient reagent equilibration (disrupted binding kinetics), over-washing (stripped MPO-antibody complexes), or expired HRP substrate (inactive enzyme). The solution is to reprocess fresh samples with immediate cold storage, equilibrate all reagents for 30 minutes, reduce wash cycles to 2 with a 20-second soak time, and replace the HRP substrate with a fresh vial. High background staining is the second most common issue, caused by cross-contamination (reused pipet tips), incomplete washing (unbound Streptavidin-HRP), or over-incubation with HRP substrate (substrate saturation). To resolve this, use fresh sterile pipet tips for all steps, increase wash cycles to 4 with a 40-second soak time, and strictly adhere to the kit’s substrate incubation time. Non-linear standard curves are almost always caused by improper MPO standard handling: repeated freeze-thaw cycles, incorrect dilution (using water instead of kit-provided standard diluent), or pipetting errors. The fix is to use aliquoted, freshly thawed MPO standard, dilute only with the kit’s standard diluent (formulated to mimic biological fluid matrix and preserve MPO), and verify pipet accuracy with a calibration check. High inter-well variability (CV >15%) stems from uneven mixing during incubation or inconsistent pipetting; the solution is to use a low-frequency oscillator for continuous mixing and pipet all samples/standards slowly and uniformly to the bottom of the wells. This targeted troubleshooting guide addresses the MPO-specific and assay-related errors unique to the Abbkine KTE61560 kit, ensuring researchers quickly resolve issues and generate high-quality data.
In conclusion, the Abbkine Human Myeloperoxidase (MPO) ELISA Kit (Cat. No. KTE61560) is the gold-standard tool for quantitative human MPO detection, and this comprehensive practical guide and methodological framework unlocks the kit’s full performance potential by addressing the unique biochemical challenges of MPO research (product link: https://www.abbkine.com/product/human-myeloperoxidase-mpo-elisa-kit-kte61560/). From MPO-specific sample preprocessing that prevents enzymatic degradation to optimized reagent handling, core workflow tuning, strategic storage management, rigorous data validation, and targeted troubleshooting, every step in this guide is designed to eliminate analytical error and generate reproducible, publication-quality MPO quantification data. The kit’s high specificity (no cross-reactivity with MPO analogues), validated standard curve (R²=0.9921), and biotin-Streptavidin-HRP signal amplification system make it an indispensable asset for research spanning innate immunity, neutrophil biology, inflammatory disease, and clinical translational science. By following this practical guide, researchers of all experience levels can leverage the full power of the Abbkine KTE61560 MPO ELISA kit to answer critical questions about MPO’s role in health and disease, advancing the field of immunology and laying the groundwork for the development of MPO-targeted diagnostic and therapeutic strategies for inflammatory and infectious disorders. For all human MPO quantitative detection needs—from basic immune function research to clinical biomarker validation—the Abbkine KTE61560 Human Myeloperoxidase ELISA Kit is the definitive choice, with a practical methodology that makes high-quality MPO quantification accessible to labs of all sizes.











