Abbkine ERα Polyclonal Antibody (ABP0086): A Precision Tool for Non-Phosphorylated Y537 ERα Detection in Reproductive and Cancer Biology
Estrogen receptor alpha (ERα, encoded by ESR1) is a ligand-activated transcription factor with pivotal roles in mammalian sexual development, reproductive function, bone metabolism, and cellular homeostasis—while also standing as a critical diagnostic and prognostic biomarker for estrogen receptor-positive (ER+) breast cancer, endometrial cancer, and other hormone-driven malignancies. The study of ERα’s functional regulation, particularly its post-translational modification at key residues like Y537, demands highly specific antibodies that can distinguish distinct molecular forms of the receptor and perform reliably across diverse experimental assays. Yet many commercial ERα antibodies suffer from critical limitations: cross-reactivity with ERβ, inability to discriminate phosphorylation states of Y537, narrow species reactivity, or poor performance in formalin-fixed paraffin-embedded (FFPE) tissue analysis. The Abbkine ERα Polyclonal Antibody (Cat. No. ABP0086) (product link: https://www.abbkine.com/product/er%ce%b1-polyclonal-antibody-abp0086/) addresses these industry pain points, engineered as an affinity-purified rabbit polyclonal antibody raised against the non-phosphorylation site of Y537 in human ERα. This reagent delivers exceptional specificity for endogenous non-phosphorylated ERα, validated cross-species reactivity with human, mouse, and rat, and robust performance across Western Blot (WB), Immunohistochemistry (IHC), Immunofluorescence (IF), and ELISA—establishing it as a gold-standard tool for ERα research in basic and translational life science.
Targeted immunogen design at the Y537 non-phosphorylation site is the defining molecular advantage of the Abbkine ABP0086 ERα Polyclonal Antibody, filling a critical gap in tools for studying ERα post-translational regulation. Unlike generic ERα antibodies that target highly conserved DNA-binding or ligand-binding domains (and often cross-react with ERβ) or phospho-specific ERα antibodies that only detect the activated phosphorylated form of Y537, this antibody is generated using a synthetic peptide derived specifically from the non-phosphorylation site of Y537 in human ERα. The Y537 residue is a well-characterized regulatory hotspot for ERα: its phosphorylation modulates receptor nuclear localization, transcriptional activity, and resistance to endocrine therapies (e.g., tamoxifen) in ER+ breast cancer. By selectively recognizing the non-phosphorylated form of this residue, the ABP0086 antibody enables researchers to directly investigate the basal, unactivated state of ERα and its dynamic shift to phosphorylated Y537 in response to estrogen signaling, growth factors, or environmental stress—an experimental capability unavailable with most commercial ERα reagents. The antibody is further refined via epitope-specific affinity chromatography from rabbit antiserum, a rigorous purification process that depletes non-specific immunoglobulins and enriches for high-affinity ERα-binding clones, ensuring it exclusively detects endogenous ERα at its expected molecular weight of 66kD with no off-target bands or cross-reactivity with ERβ or other nuclear receptors.
Validated cross-species reactivity with human, mouse, and rat ERα positions the Abbkine ABP0086 ERα Polyclonal Antibody as a universal tool for interdisciplinary research, eliminating the inefficiencies of species-specific antibody validation and optimization. A core challenge in ERα research is the need to translate findings from animal models (e.g., mouse breast cancer models, rat reproductive development studies) to human clinical samples— a process that often requires separate, species-optimized antibodies, introducing experimental variability and increasing research costs. The ABP0086 antibody bypasses this issue with robust, validated reactivity across all three key model organisms, allowing researchers to use a single reagent for in vitro human cell line studies, in vivo mouse/rat animal models, and ex vivo human FFPE tissue analysis. This cross-species consistency ensures direct comparability of ERα expression and localization data across experimental systems, a critical requirement for translational research linking basic ERα biology to human disease. For example, a lab investigating ERα’s role in breast cancer progression can use the ABP0086 antibody to profile non-phosphorylated Y537 ERα in mouse mammary tumor models and immediately validate those findings in human breast cancer FFPE samples—no assay re-optimization or antibody replacement required.
Multi-assay optimized performance and application-specific practical guidelines make the Abbkine ABP0086 ERα Polyclonal Antibody a versatile workhorse for both qualitative and quantitative ERα research, with clear, researcher-tailored starting dilutions and validated experimental conditions that minimize trial-and-error optimization. The antibody is fully validated for four core immunoassays—WB, IHC, IF, and ELISA—with suggested starting dilutions of 1:500–1:2000 (WB), 1:100–1:300 (IHC), 1:200–1:1000 (IF), and 1:10000 (ELISA), and real-world experimental data confirming its exceptional performance at these ranges:
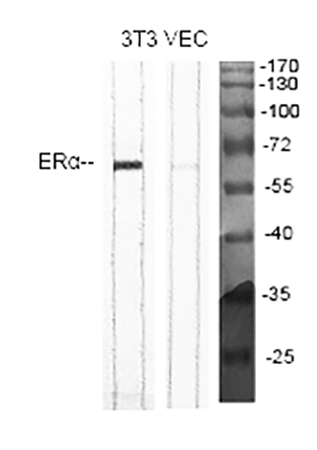
- WB: A 1:2000 dilution produces a sharp, single 66kD band for ERα in 3T3 and VEC cell lysates, with no smearing or background signal—this high dilution minimizes antibody usage and maximizes throughput for high-volume cell line screening.
- IHC: A 1:200 dilution delivers crisp, nuclear-specific staining in human, mouse, and rat lung FFPE tissues, with the critical validated step of sodium citrate buffer (pH 6.0) antigen retrieval at >98°C for 20 minutes; this step is non-negotiable for FFPE tissue analysis, as it unmasks the ERα epitope masked by formalin cross-linking, a practical insight built from the antibody’s experimental validation.
- IF: A 1:200 dilution yields bright, specific red fluorescence for ERα in rat lung tissue (paired with a Cy3-conjugated secondary antibody at 1:300), with clear nuclear localization and no diffuse cytoplasmic background—ideal for studying ERα subcellular dynamics in fixed cells and tissues.
- ELISA: An ultra-high 1:10000 dilution enables sensitive quantitative detection of endogenous non-phosphorylated ERα in biological fluids and cell lysates, making it suitable for high-throughput quantification of ERα expression levels in response to hormonal or therapeutic treatment.
These application-specific guidelines eliminate the guesswork common with generic ERα antibodies, allowing researchers to generate publication-quality data from the first assay.
Optimized liquid formulation and robust storage stability ensure the Abbkine ABP0086 ERα Polyclonal Antibody maintains peak performance over its shelf life, with practical handling guidelines that reduce experimental variability and reagent waste—critical considerations for long-term research projects and high-throughput labs. The antibody is supplied as a ready-to-use liquid solution at a concentration of 1 mg/ml, formulated in a gold-standard buffer of PBS containing 50% glycerol, 0.5% BSA, and 0.02% sodium azide. This formulation is engineered for maximum antibody stability: 50% glycerol prevents ice crystal formation during freezing, eliminating antibody denaturation from repeated freeze-thaw cycles; BSA acts as a protein stabilizer to preserve the antibody’s tertiary structure and epitope-binding capacity; and sodium azide serves as a mild preservative safe for all in vitro immunoassays. The antibody is stable for one year at -20°C from shipment, with two key practical handling steps to maximize recovery and performance: centrifuge the vial after thawing and before opening to collect antibody adhered to the vial wall, and aliquot the antibody into small 5–10μl volumes upon first use to avoid repeated freeze-thawing (a major cause of reduced binding affinity in polyclonal antibodies). Unlike lyophilized ERα antibodies that require reconstitution (a step that introduces volume and concentration variability), the liquid formulation of the ABP0086 antibody allows for immediate use upon thawing, reducing assay setup time and ensuring consistent antibody concentration across all experiments.
The Abbkine ABP0086 ERα Polyclonal Antibody’s ability to detect endogenous non-phosphorylated Y537 ERα makes it an indispensable tool for translational research into hormone-driven cancer and endocrine therapy resistance, addressing a critical unmet need in oncology research. ERα is the primary target of endocrine therapies (e.g., selective estrogen receptor modulators, aromatase inhibitors) for ER+ breast cancer, and resistance to these therapies is a major clinical challenge—one linked to post-translational modifications of ERα, including phosphorylation at Y537. Non-phosphorylated Y537 ERα represents the basal state of the receptor, and its phosphorylation is a key step in receptor activation and the development of therapy resistance. Most commercial ERα antibodies either do not distinguish phosphorylation states or only detect the phosphorylated form, leaving researchers unable to quantify the basal non-phosphorylated pool and its dynamic changes in cancer cells. The ABP0086 antibody fills this gap, enabling the quantification and localization of non-phosphorylated Y537 ERα in human breast cancer FFPE samples, mouse tumor models, and cancer cell lines—research that can uncover new mechanisms of therapy resistance and identify novel biomarkers for patient stratification. Beyond oncology, this antibody is a powerful tool for studying ERα’s role in normal physiology, including sexual development, bone metabolism, and reproductive function, where the basal non-phosphorylated state of the receptor is critical for maintaining cellular homeostasis.
Exceptional specificity for endogenous ERα and lack of cross-reactivity with ERβ further solidify the Abbkine ABP0086 ERα Polyclonal Antibody as a gold-standard reagent, eliminating the data bias and experimental error common with cross-reactive ERα antibodies. Estrogen signaling is mediated by two closely related receptors, ERα and ERβ, which share significant structural homology in their DNA-binding and ligand-binding domains. Many commercial ERα antibodies target these conserved domains, leading to cross-reactivity with ERβ—an issue that skews data in tissues and cell lines where both receptors are expressed (e.g., breast, ovary, brain). The ABP0086 antibody, by contrast, targets the non-phosphorylation site of Y537 in ERα, a residue that is not conserved in ERβ, ensuring it exclusively detects ERα with no cross-reactivity. This high specificity is critical for studying the distinct functional roles of ERα and ERβ in estrogen signaling, as it allows researchers to attribute experimental results directly to ERα without confounding signals from ERβ. The antibody’s ability to detect endogenous levels of ERα (not just overexpressed recombinant protein) is an additional key advantage, as it enables research on the receptor in its native cellular context—an essential requirement for translating findings to human physiology and disease.
Cost-effectiveness and high-performance scalability make the Abbkine ABP0086 ERα Polyclonal Antibody an ideal choice for labs of all sizes, from small academic research groups to large translational science and biotech teams. Priced at $229 for 100μl of 1 mg/ml antibody, the ABP0086 reagent offers an unbeatable cost-performance ratio due to its high suggested working dilutions: a single 100μl vial can support hundreds of WB assays (at 1:2000), dozens of IHC/IF slides (at 1:200–1:300), and thousands of ELISA wells (at 1:10000). This scalability eliminates the need for frequent antibody reordering and reduces the per-assay cost of ERα detection, a critical benefit for labs with limited research budgets or high-throughput experimental needs. Unlike high-end commercial ERα antibodies that offer similar specificity but at a significantly higher price point, the ABP0086 antibody delivers laboratory-grade performance at an accessible cost, democratizing access to high-quality ERα detection tools for researchers worldwide.
In conclusion, the Abbkine ERα Polyclonal Antibody (Cat. No. ABP0086) (product link: https://www.abbkine.com/product/er%ce%b1-polyclonal-antibody-abp0086/) redefines the standard for ERα research, combining targeted design at the non-phosphorylation Y537 site, exceptional specificity for endogenous ERα, validated cross-species reactivity (human, mouse, rat), and robust multi-assay performance (WB, IHC, IF, ELISA). Its application-specific practical guidelines, optimized liquid formulation, and long-term storage stability eliminate experimental variability and minimize trial-and-error optimization, while its ability to detect the basal non-phosphorylated form of ERα fills a critical gap in tools for studying ERα post-translational regulation and hormone-driven cancer resistance. Whether used for basic research into ERα’s role in reproductive development and bone metabolism or translational research into ER+ cancer biomarkers and therapy resistance, the ABP0086 antibody is a precision, versatile, and cost-effective tool that delivers publication-quality results across all experimental systems. For researchers committed to rigorous ERα research, this antibody stands as the gold-standard choice for non-phosphorylated Y537 ERα detection in health and disease.
Key Longtail Keywords for Search Visibility
Abbkine ERα Polyclonal Antibody ABP0086, Non-phosphorylation Y537 ERα Antibody, Human Mouse Rat ERα Polyclonal Antibody, WB IHC IF ELISA ERα Detection Antibody, Endogenous ERα Polyclonal Antibody, ESR1 Estrogen Receptor Alpha Antibody, FFPE Tissue ERα Polyclonal Antibody, ERα Antibody for Breast Cancer Research











